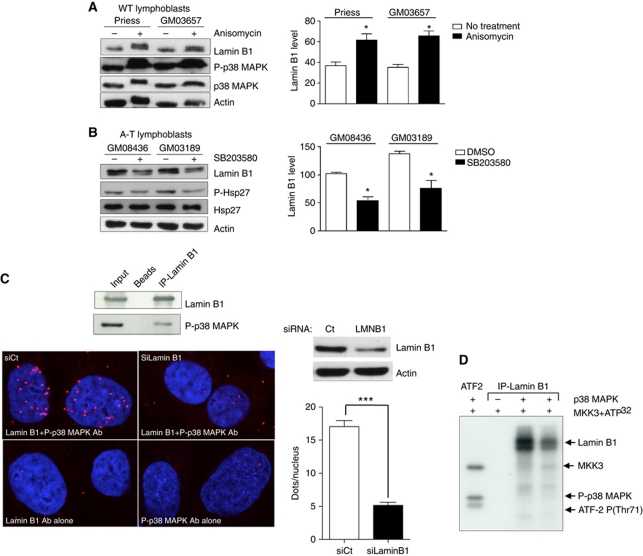
Figure 7.
Impact of p38 MAPK on lamin B1 levels and interactions between p38 MAPK and lamin B1. Modifications of lamin B1 levels after anisomycin (A) or SB203580 (B) treatment. Left panels: western blot analysis after 24 h of anisomycin (10 μg/ml) or SB203580 (10 μM) treatment. Right panels: quantification of lamin B1 after anisomycin (A) or SB203580 (B) treatment. Immunoblotting of P(T180-Y182)-p38 MAPK and P-(Ser82)-Hsp27 protein, a substrate of p38 MAPK, was performed to confirm the efficiency of anisomycin and inhibitor SB203580, respectively, on p38 MAPK activity. (C) Upper panel: co-immunoprecipitation of lamin B1 (overexpressed 48 h before protein extraction) and endogenous P-p38 MAPK. Lower panels: in-situ interactions between endogenous lamin B1 and activated p38 MAPK monitored by the proximity ligation assay (PLA) using the anti-P(T180/Y182)-p38 MAPK and lamin B1 (bottom panel) antibodies. Proximal locations between the two proteins were observed as red fluorescent dots. Right upper panel: a western blot showing the efficiency of lamin B1 siRNA 48 h after treatment. Right lower panel: quantification of in-situ PLA in cells transfected with negative control siRNA (siCtrl) or with lamin B1 siRNA (siLMNB1). Each value represents the mean number of dots in >155 nuclei. *Represents a statistically significant difference (P<0.05). ***Represents P<0.0001 (t-test). The error bars denote the s.e.m. (D) In-vitro phosphorylation of lamin B1 by p38 MAPK on SV-40 fibroblasts. The kinase activity of p38 MAPK on immunoprecipitated lamin B1 protein from two WT lymphoblasts protein extracts (Priess and GM03657) was evaluated by a radioactive assay. In the presence of MKK3 (a p38 MAPK activator) and 32PATP, p38 MAPK phosphorylated lamin B1 in vitro (lanes 3 and 4). In the first lane, ATF2-P(T71), a specific substrate of p38 MAPK, served as a positive control of p38 MAPK activity. In the second lane, no lamin B1 phosphorylation was detected in the absence of p38 MAPK.