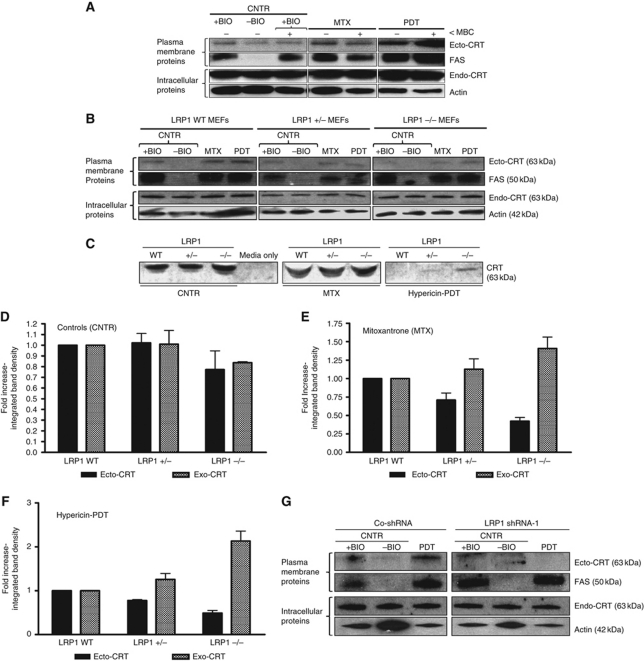
Figure 7.
Ecto-CRT induced by phox-ER stress docks on the surface via the LRP1 molecule. (A) Ecto-CRT induced by phox-ER stress is not dependent on lipid rafts. T24 cells were preincubated with 2 μM of MBC for 1 h and then treated with a medium PDT dose, MTX (1 μM for 1 h) or left untreated (CNTR). They were recovered 5 min post PDT. Surface proteins were biotinylated and immunoblotted. In (A), (B), and (G), ‘+BIO’ indicates controls exposed to buffer with biotin and ‘−BIO’ indicates controls exposed to buffer without biotin (negative control). (B–F) Ecto-CRT retention on ER-stressed cancer cells is dependent on the level of LRP1. MEF cells containing levels of 100% LRP1 (LRP1 WT), 50% LRP1 (LRP1+/−), or 0% LRP1 (LRP−/−) were treated with a low PDT dose or MTX (1 μM for 4 h in serum-free media) followed by recovery in serum-free media after 1 h post PDT. Surface proteins were biotinylated and immunoblotted (B) as detailed in (A). Simultaneously, the conditioned media (CM) derived from these cells were concentrated and immunoblotted (C). The integrated density of CRT protein bands in (B) and (C) was quantified by Image J software for untreated (D), MTX-treated (E), and PDT-treated (F) conditions: where black is for ecto-CRT bands in the immunoblots of biotinylated surface proteins and grey is for ‘exo-CRT’ bands in the immunoblots of concentrated CM. Data have been normalized to the respective LRP1 WT values. Fold change values are means of three independent experiments±s.e.m. (G) Induction of ecto-CRT by phox-ER stress is reduced by LRP1 shRNA. CO-shRNA CT26 cells and CT26 expressing LRP1 shRNA 1 were treated with medium PDT dose and recovered 1 h post PDT followed by surface biotinylation as detailed in (A).