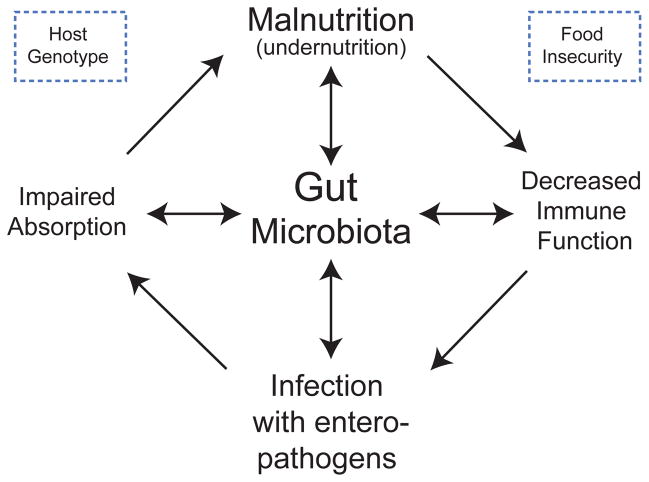
Fig. 1. A schematic of envisioned interrelationships between the gut microbiota, the immune system and diet that underlies the development of malnutrition.
Under-nutrition is associated with a variety of defects in the innate and adaptive immune system that, in turn, are associated with increased predisposition to diarrheal illnesses. Recurrent (enteric) infections predispose to macro- and micronutrient deficiencies, as well as impaired intestinal mucosal barrier function 77. These factors lead to a cycle of further susceptibility to infection and worsening nutritional status. A confounding problem is that vaccines designed to protect children from certain pathogens (including enteropathogens) exhibit poor efficacy in areas of the world where poor nutrition is rampant 74. A testable hypothesis is that the gut microbiota may contribute to disease risk and pathogenesis through effects on nutrient processing and absorption, and on immune function. These interrelationships are illustrated in the Figure. Diet shapes gut microbial community structure and function while the microbiota adapts in ways that promote nutrient processing; the ability of the microbiota to process a given diet affects the nutrient and energetic value of that diet. The microbiota and immune systems co-evolve: malnutrition affects the innate and adaptive immune system as well as the microbiota. The microbiota serves as a barrier to enteropathogen infection; this barrier function may be disrupted by malnutrition as well as by perturbations in immune system function. The microbiota affects nutrient processing and nutrient distribution to the host, including the expression of host genes involved in nutrient transport and metabolism.