Abstract
Mycobacterium marinum is a waterborne mycobacterial pathogen. Due to their common niche, protozoa likely represent natural hosts for M. marinum. We demonstrate that the ESX-1 secretion system is required for M. marinum pathogenesis and that M. marinum utilizes actin-based motility in amoebae. Therefore, at least two virulence pathways used by M. marinum in macrophages are conserved during M. marinum infection of amoebae.
TEXT
Mycobacterial pathogens are responsible for some of the leading causes of death by infectious disease. The majority of these deaths are caused by mycobacterial species within the Mycobacterium tuberculosis complex (MTC) (3). However, mycobacterial species in the environment, including atypical or nontuberculous mycobacteria (NTM), pose an emerging disease threat (19). Little is understood about the basic molecular virulence mechanisms employed by environmental mycobacterial pathogens.
Mycobacterium marinum is a waterborne pathogen that causes a tuberculosis (TB)-like infection in ectotherms and is an occasional opportunistic human pathogen (21). M. marinum is related to M. tuberculosis and is used to model aspects of MTC pathogenesis (4, 7, 21, 23, 27). Free-living amoebae (FLA), including Acanthamoeba castellanii, are professional phagocytes (12). Pathogenic bacteria, including M. marinum and other NTM, have been recovered from samples of water colonized by free-living amoebae (8, 26). Several mycobacterial species are established amoeba-resistant bacteria (ARB) and resist destruction by FLA (1, 6, 12, 17, 18). It has been posited that protozoa serve as a reservoir for mycobacteria in the environment (17).
It is probable that M. marinum naturally interacts with protozoa, including A. castellanii, that share an environmental niche. It was demonstrated that M. marinum are pathogenic to A. castellanii (6, 17, 20, 28). The molecular mechanisms underlying this interaction have not been well established.
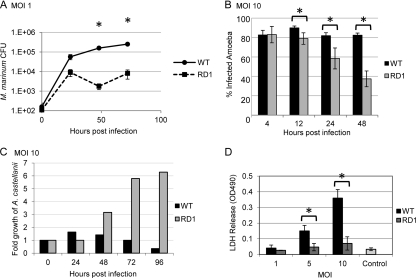
We hypothesized that virulence mechanisms required for infection of macrophages by M. marinum would also be required for pathogenesis of A. castellanii. The ESX-1 protein secretion system is required by mycobacteria and other Gram-positive pathogens to cause disease (2, 11, 13, 16, 24). Amoebae were infected (multiplicity of infection [MOI] of 1) with either the wild-type (WT) strain or an attenuated RD1 deletion (ΔRD1) strain of M. marinum, which bears a deletion in components and substrates of the ESX-1 system (Fig. 1A) (27). M. marinum replicated roughly three logs in A. castellanii over the 72-h experiment, with an average generation time of 7 h. The ΔRD1 strain replicated approximately two logs in A. castellanii over the 72-h experiment, with an average generation time of 12 h. Following an early rapid growth phase for both strains, the bacteria were maintained at relatively constant levels throughout the experiment. However, the ΔRD1 strain failed to reach the levels of growth achieved by the wild-type strain (Fig. 1A). Significant differences in growth between the WT and ΔRD1 strains were observed at 48 (P < 0.035) and 72 (P < 0.002) hours postinfection (hpi) (Fig. 1A).
Fig 1.
M. marinum pathogenesis within A. castellanii requires the ESX-1 secretion system. (A) M. marinum replicates and persists within A. castellanii, while the M. marinum strain lacking ESX-1 is attenuated. Amoebae were infected (MOI of 1) and treated with gentamicin (150 μg/ml). Amoebae were lysed and bacteria were plated for CFU at the indicated times. Error bars represent standard deviations. Asterisks represent statistically significant differences based on an unpaired tailed Student t test (P < 0.05) (48 hpi, P = 0.035; 72 hpi, P = 0.002). (B) Infection by M. marinum results in a stable percentage of infected amoebae, while the population of infected amoebae decreased over time in the absence of the ESX-1 system. A. castellanii was infected with M. marinum and ΔRD1 M. marinum expressing DsRed at an MOI of 10. Monolayers were imaged using a 20× objective on a Zeiss AxioObserver microscope. The percentage of infected amoebae was established after counting >100 cells in each of five different fields at the indicated time points (12 h, P = 0.005; 24 h, P = 0.010; 48 h, P = 6.547 × 10−5). (C) Infection of A. castellanii results in a relatively constant number of amoebae over time, while infection by M. marinum lacking ESX-1 leads to amoeba growth. At each time point following infection, the amoebae were counted and the number was normalized to the initial number of amoebae plated. See Fig. S3 in the supplemental material for amoeba counts. (D) Infection with M. marinum results in amoeba lysis in an ESX-1-dependent manner. MOI of 5, P = 0.009; MOI of 10, P = 0.001. The control is uninfected amoebae after 72 h of incubation.
Next, we infected A. castellanii with WT or ΔRD1 M. marinum expressing DsRed at an MOI of 1, 5, and 10 and monitored bacterial uptake and survival. We performed fluorescence microscopy and determined the percentage of amoebae infected with M. marinum. At each time point, the numbers of infected amoebae were counted and compared to the total number of amoebae in that field (see Fig. S1 in the supplemental material). Five fields of approximately 100 amoebae were counted at each time point. We found that with increasing MOI, the fraction of infected amoebae increased (Fig. 1B; see also Fig. S2 in the supplemental material). At 4 hpi, an MOI of 1 resulted in 20 to 25% of A. castellanii cells becoming M. marinum infected (see Fig S2). At MOIs of 5 and 10, roughly 60% and 80% of A. castellanii cells became infected, respectively (Fig. 1B; see also Fig S2). Importantly, our data indicate that once M. marinum is phagocytosed by amoebae, the proportion of A. castellanii cells infected remains constant for at least 48 h (Fig. 1B). In contrast, although the ΔRD1 strain is not defective for uptake relative to the wild-type strain (Fig. 1B, 4 hpi), the population of infected amoebae significantly decreased over time at all MOIs tested relative to the wild-type infection (Fig. 1B; 12 hpi, P = 0.005; 24 hpi, P = 0.010; 48 hpi, P = 6.547 × 10−5).
It was previously reported that Legionella pneumophila infection of A. castellanii results in amoeba lysis and reduced viability (5, 9, 28). To determine if we observed a similar decrease in amoeba viability, we counted amoebae during infection with wild-type M. marinum at an MOI of 10 and normalized the total number of amoebae each day to the number plated at time zero. The total number of amoebae remained constant when infected with wild-type M. marinum (Fig. 1C; see also Fig. S3 in the supplemental material). In contrast, infection with ΔRD1 M. marinum resulted in amoeba replication over time (Fig. 1C; see also Fig. S3). Because the amoebae infected with the attenuated strain continue to replicate during the course of the infection, growth arrest is likely due to infection with wild-type M. marinum rather than nutrient exhaustion. To confirm that we were observing amoeba death, we performed a lactate dehydrogenase (LDH) release assay at various MOIs 72 hpi. Infection with increasing MOI of wild-type M. marinum resulted in significantly increased amoebal lysis as measured by LDH release (Fig. 1D; MOI of 5, P = 0.009; MOI of 10, P = 0.001). Importantly, we did not observe detectable lysis during infection by the ΔRD1 deletion strain of M. marinum at any MOI tested or of uninfected amoebae.
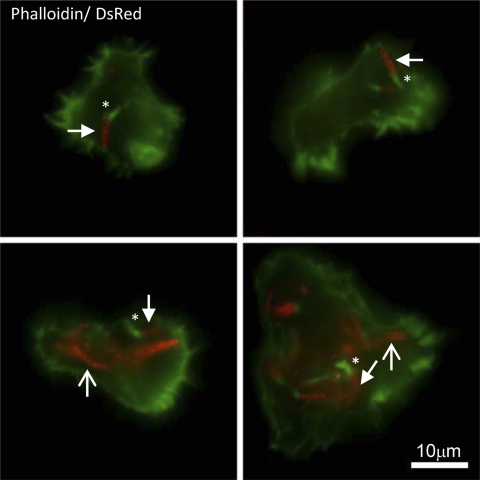
One of the features of M. marinum pathogenesis is the ability of the bacteria to access the macrophage cytosol and utilize host actin for motility (22). Interestingly, M. marinum fails to exhibit actin-based motility in the soil amoeba Dictyostelium discoideum and instead escapes via nonlytic ejection (14, 15). To determine if M. marinum forms actin tails in A. castellanii, we infected amoebae at an MOI of 5 and visualized actin tail formation using immunofluorescence microscopy. We observed actin tail formation by wild-type M. marinum at approximately 22 hpi (Fig. 2). As was reported in macrophages, we observed that only a subset of mycobacteria form actin tails (22). Similarly, a single amoeba cell infected with multiple bacteria harbors bacteria with and without tails. We were unable to visualize actin tails in bacteria lacking the ESX-1 system (data not shown).
Fig 2.
Wild-type M. marinum forms actin tails in A. castellanii. Florescence microscopy demonstrating actin tail formation by M. marinum at 22 hpi, MOI of 5. M. marinum is expressing DsRed, and Alexa 488 phalloidin is green. Four representative images are shown. The scale bar is 10 μm. Images were acquired with an Evolution QEi charge-coupled device (CCD) (Media Cybernetics) on a Nikon Eclipse TE300 microscope (60× objective) using IPLab software (Scanalytics). Examples of bacteria bearing tails are indicated with filled arrows. Tails are indicated with asterisks. Examples of bacteria without tails are indicated with open arrows.
In conclusion, we demonstrate that two virulence mechanisms used by M. marinum to infect macrophages are also required for pathogenesis of the amoeba A. castellanii under laboratory conditions. The M. marinum strain lacking the ESX-1 secretion system was attenuated for growth in amoebae, as previously shown in macrophages and zebrafish (7, 10, 25, 27). Moreover, infection by M. marinum resulted in lysis of the amoeba host in an ESX-1-dependent manner. We observed actin tail formation by M. marinum but were unable to observe actin tail formation in the ESX-1-deficient strains (data not shown), consistent with the requirement of ESX-1 for phagosomal escape by M. marinum (22, 23). Our findings contribute to the basic molecular understanding of the interaction between A. castellanii and M. marinum.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Jeffery S. Cox, members of the Cox lab, Eric J. Brown, and Matthew Champion for helpful discussion of this work. We thank the Notre Dame Integrated Imaging Facility (ndiif.nd.edu) for microscopy support.
This work was supported in part by the NIH under Ruth L. Kirschstein Research Service Award A105155 to P.A.D.C. and Capitalization Funds from the University of Notre Dame to P.A.D.C. G.M.K. is supported by the GLOBES fellowship program, funded by an IGERT training grant from the National Science Foundation (grant no. 504495).
Footnotes
Published ahead of print 13 January 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Adekambi T, Ben Salah S, Khlif M, Raoult D, Drancourt M. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72:5974–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burts ML, Williams WA, DeBord K, Missiakas DM. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc. Natl. Acad. Sci. U. S. A. 102:1169–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cangelosi GJC-C, Behr M, Bull T, Stinear T. 2004. Biology of waterborne pathogenic mycobacteria, p 39–54 In Pedley S, Bartram J, Rees G, Dufour A, Cotruvo JA. (ed), Pathogenic mycobacteria in water: a guide to public health consequences, monitoring and management. World Health Organization, Geneva, Switzerland [Google Scholar]
- 4. Chan K, et al. 2002. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc. Natl. Acad. Sci. U. S. A. 99:3920–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cirillo JD, Falkow S, Tompkins LS. 1994. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 62:3254–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cirillo JD, Falkow S, Tompkins LS, Bermudez LE. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosma CL, Humbert O, Ramakrishnan L. 2004. Superinfecting mycobacteria home to established tuberculous granulomas. Nat. Immunol. 5:828–835 [DOI] [PubMed] [Google Scholar]
- 8. De Jonckheere JF. 1979. Pathogenic free-living amoebae in swimming pools: survey in Belgium. Ann. Microbiol. (Paris) 130B:205–212 [PubMed] [Google Scholar]
- 9. Gao L, Abu Kwaik Y. 2000. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2:1705–1719 [DOI] [PubMed] [Google Scholar]
- 10. Gao LY, et al. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 53:1677–1693 [DOI] [PubMed] [Google Scholar]
- 11. Garufi G, Butler E, Missiakas D. 2008. ESAT-6-like protein secretion in Bacillus anthracis. J. Bacteriol. 190:7004–7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greub G, Raoult D. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guinn KM, et al. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagedorn M, Rohde KH, Russell DG, Soldati T. 2009. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323:1729–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagedorn M, Soldati T. 2007. Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection. Cell. Microbiol. 9:2716–2733 [DOI] [PubMed] [Google Scholar]
- 16. Hsu T, et al. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. U. S. A. 100:12420–12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krishna-Prasad BN, Gupta SK. 1978. Preliminary report on engulfment and retention of mycobacteria by trophozoites of axenically grown Acanthamoeba castellanii Douglas. Curr. Sci. 47:245–247 [Google Scholar]
- 18. Mba Medie F, Ben Salah I, Henrissat B, Raoult D, Drancourt M. 2011. Mycobacterium tuberculosis complex mycobacteria as amoeba-resistant organisms. PLoS One 6:e20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salah IB, Ghigo E, Drancourt M. 2009. Free-living amoebae, a training field for macrophage resistance of mycobacteria. Clin. Microbiol. Infect. 15:894–905 [DOI] [PubMed] [Google Scholar]
- 20. Solomon JM, Leung GS, Isberg RR. 2003. Intracellular replication of Mycobacterium marinum within Dictyostelium discoideum: efficient replication in the absence of host coronin. Infect. Immun. 71:3578–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stamm LM, Brown EJ. 2004. Mycobacterium marinum: the generalization and specialization of a pathogenic mycobacterium. Microbes Infect. 6:1418–1428 [DOI] [PubMed] [Google Scholar]
- 22. Stamm LM, et al. 2003. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 198:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stamm LM, et al. 2005. Role of the WASP family proteins for Mycobacterium marinum actin tail formation. Proc. Natl. Acad. Sci. U. S. A. 102:14837–14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanley SA, Raghavan S, Hwang WW, Cox JS. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. U. S. A. 100:13001–13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoop EJ, et al. 2011. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis. Model Mech. 4:526–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas V, Herrera-Rimann K, Blanc DS, Greub G. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volkman HE, et al. 2004. Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2:e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan L, Cerny RL, Cirillo JD. 2004. Evidence that hsp90 is involved in the altered interactions of Acanthamoeba castellanii variants with bacteria. Eukaryot. Cell 3:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.