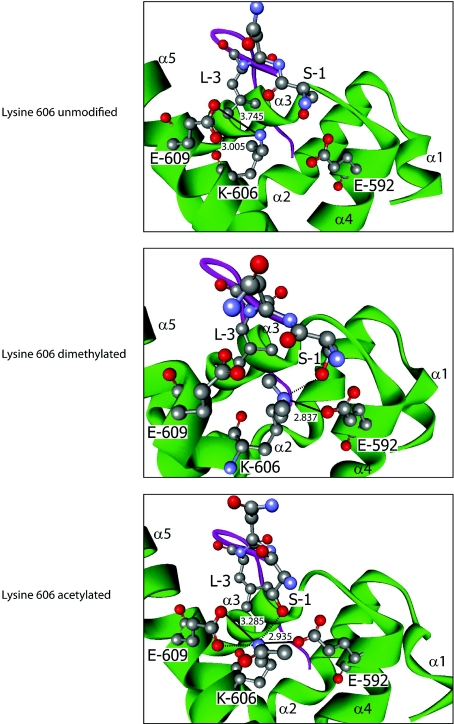
Figure 4. Effects of Lys606 modification on PABP1 PABC–PAIP2 PAM2 interaction.
Modelling of the interaction between human PABP1 PABC (green) and the PAIP2 PAM2 motif (purple) when lysine606 is dimethylated (middle panel) or acetylated (bottom panel), based on the structure derived using unmodified PABC (top panel). Where key residues are visualized, they are numbered according to their position in full-length PABP1 or within the PAIP2 PAM2 motif (1–12). α-Helices of PABC are labelled α1–5. Atoms are coloured according to the following: red, oxygen; grey, carbon; and blue, nitrogen. Hydrogen atoms are not depicted. Where appropriate, bonds are depicted as follows: salt bridge, thick black line; hydrogen bond, broken black line; numerical values, bond distance (in Å) (hydrogen bonds ≤2.5 Å). A hydrophobic pocket is formed by Met584, Ile588, Ala610 (not depicted), and Lys606 and Glu609. The hydrophobic contacts between PAM2 Leu3 and the aliphatic portions of Lys606 and Glu609 are not depicted for clarity.