Abstract
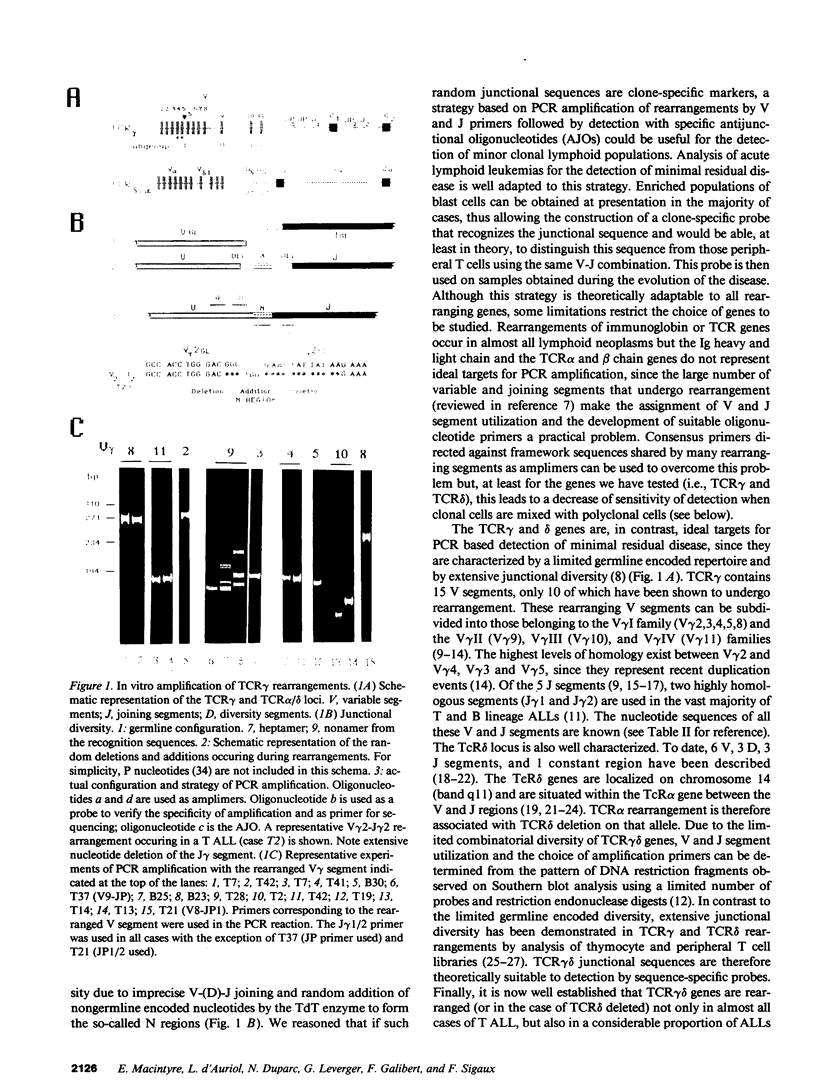
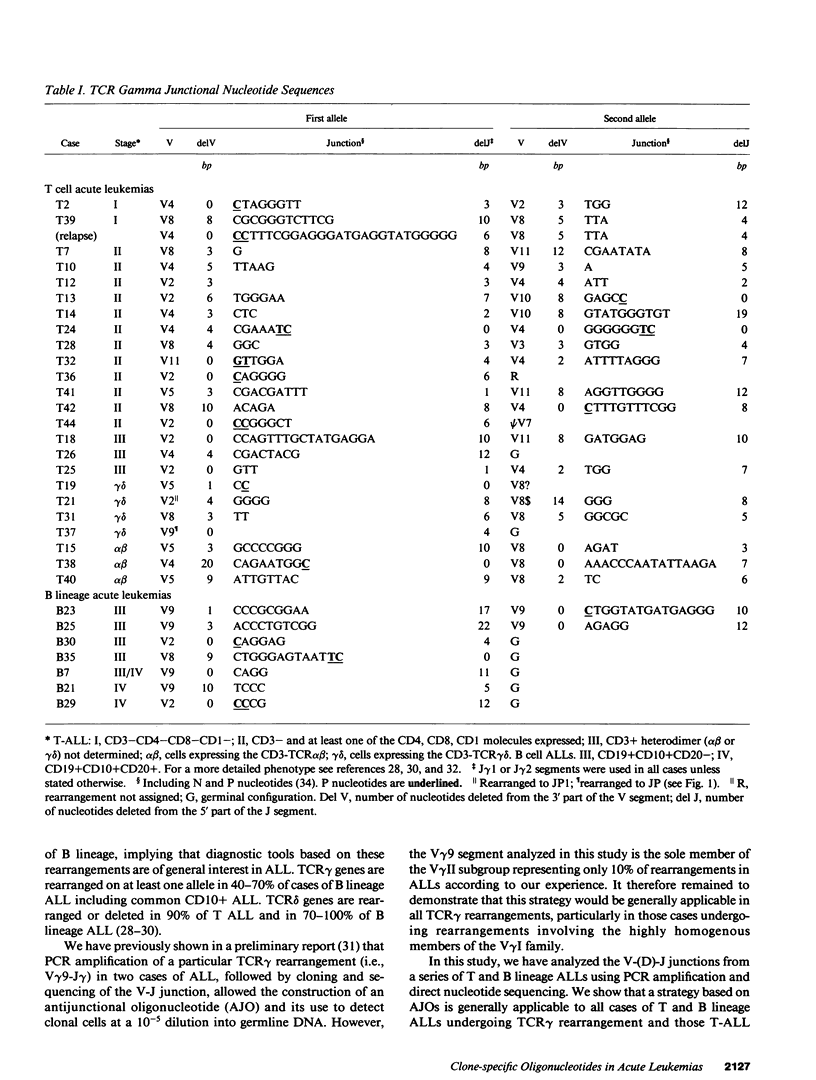
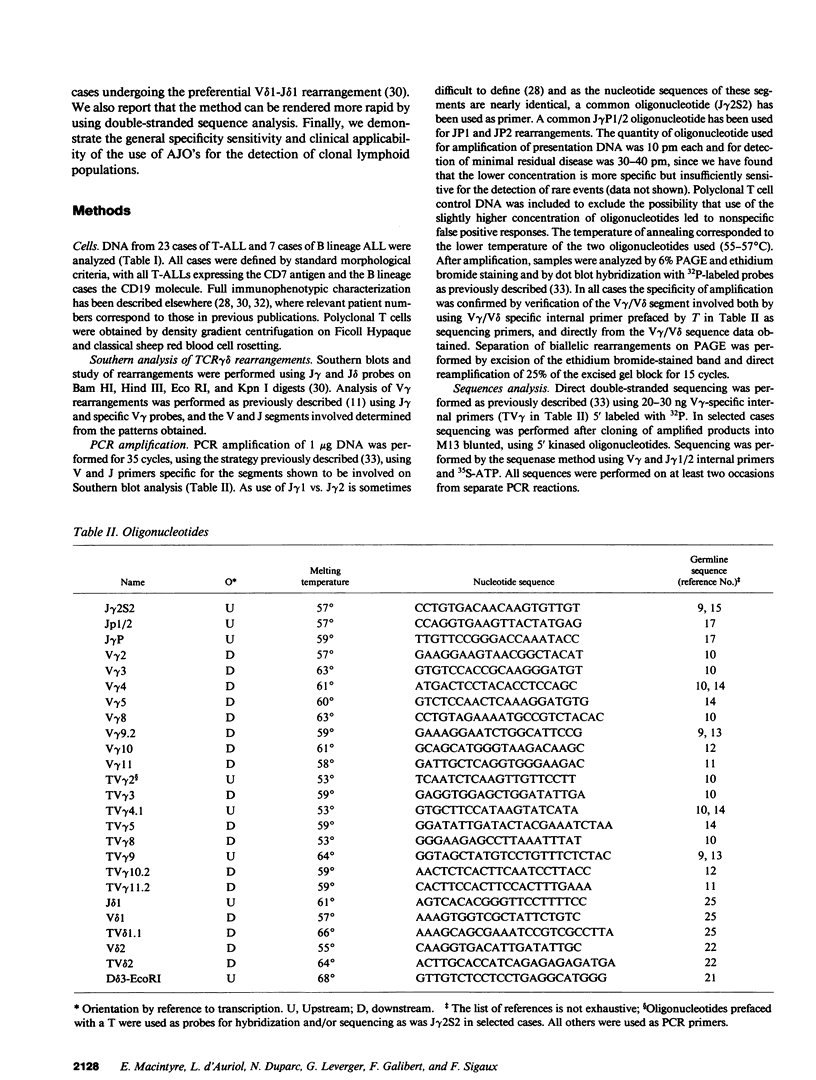
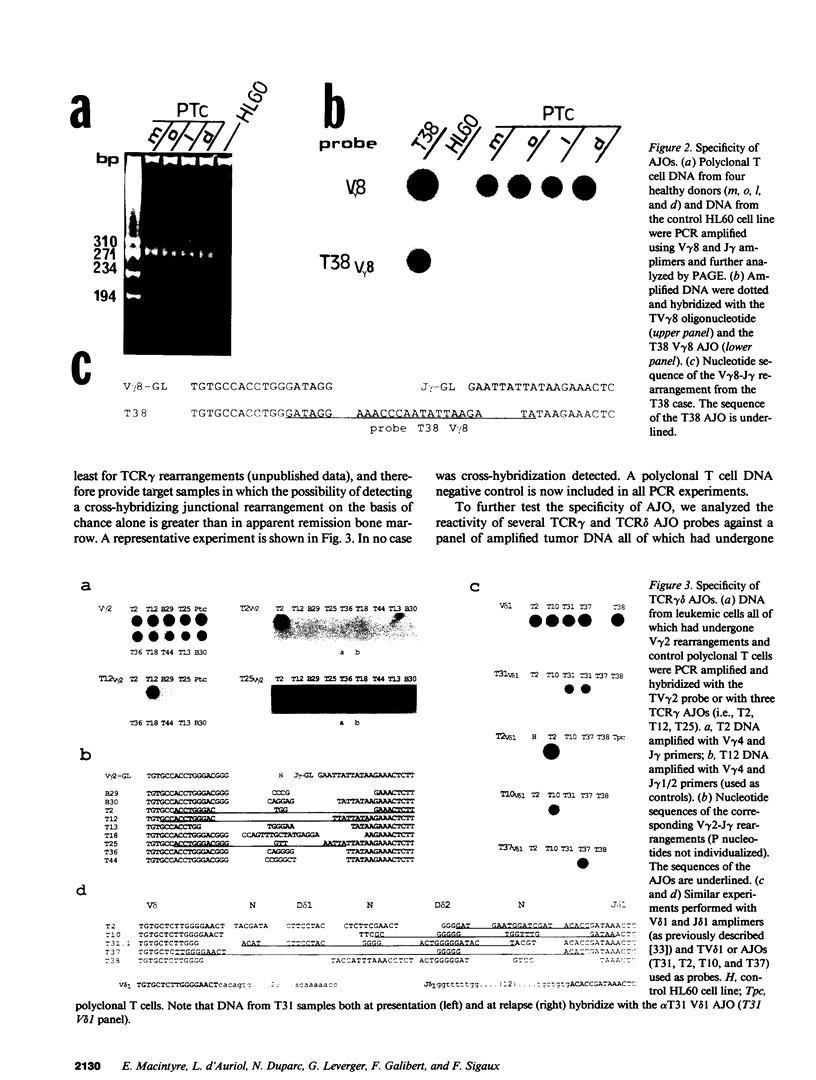
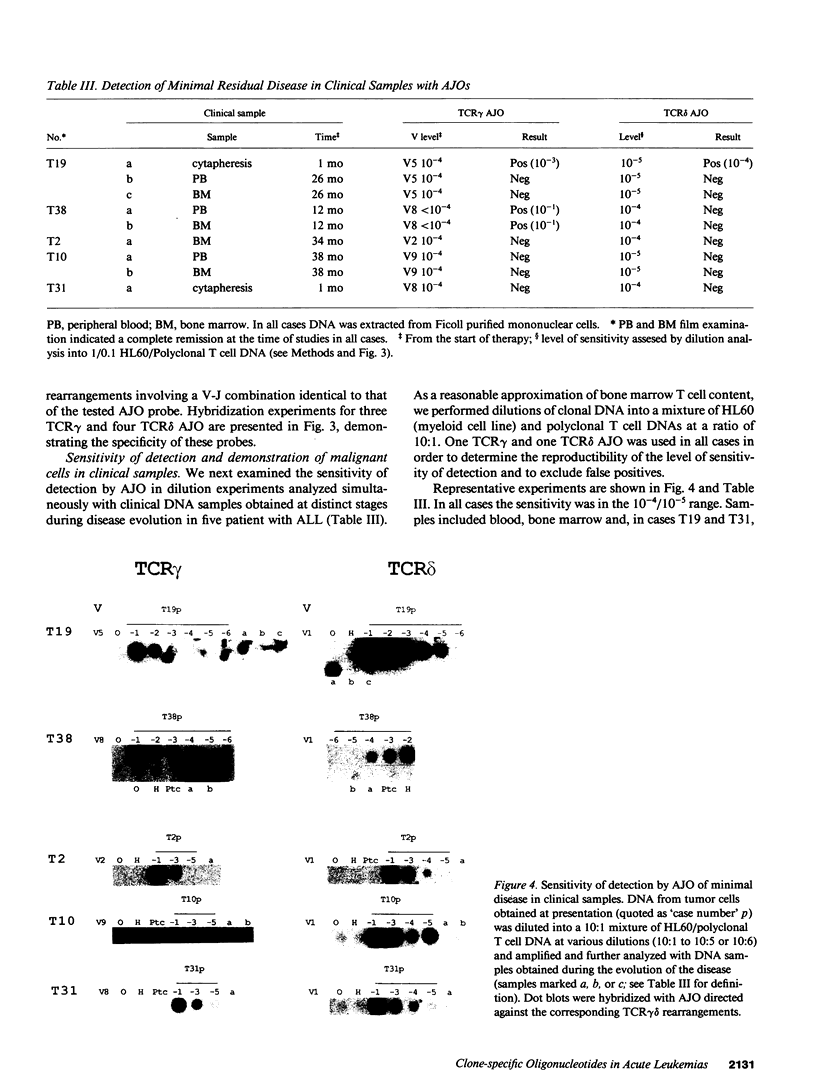
To provide a sensitive and generally applicable method to detect clonal cells in acute lymphoblastic leukemias (ALL), we have designed a new strategy based on the polymerase chain reaction (PCR) amplification of the T cell receptor gamma delta gene rearrangements found in most T and B lineage ALLs. PCR allows rapid sequencing of variable-(diversity)-joining (V-[D]-J) junctions from tumor DNA and construction of anti-junctional oligonucleotides (AJOs) used as probes to detect clonal cells in the same patient. We have defined oligonucleotides suitable for all T cell receptor (TCR) rearrangements involving functional V gamma segments. Oligonucleotides corresponding to preferential TCR delta rearrangements in T and B lineage ALLs were also used. By analysis of the nucleotide sequence of 52 V gamma-V gamma junctions from 30 cases of B and T ALLs, we demonstrate that V-J junctional sequences are clone specific in both lineages and at all stages of differentiation examined despite the frequent presence of the recently described P nucleotides. Experiments performed with TCR gamma delta AJOs on DNA from tumor cells and polyclonal T cells show that AJOs can be used to differentiate clonal cells from polyclonal T cells, distinguish between different T cell clones, and detect residual clonal populations at 10(-4)/10(-5) dilution. AJOs were also used to detect residual disease in samples from patients in clinical and morphological complete remission. Finally, rearrangement patterns were studied by classical Southern analysis in selected cases at both presentation and subsequent relapse showing absence of clonal evolution in most cases. V-(D)-J nucleotide sequences of rearrangements with an identical pattern of rearrangement at presentation and relapse were identical in all cases analyzed. We therefore describe a new, specific, and clinically useful strategy for the detection of minor clonal populations applicable in the majority of cases of ALL.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird J., Galili N., Link M., Stites D., Sklar J. Continuing rearrangement but absence of somatic hypermutation in immunoglobulin genes of human B cell precursor leukemia. J Exp Med. 1988 Jul 1;168(1):229–245. doi: 10.1084/jem.168.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Buluwela L., Williams D., White L., Rabbitts T. H. A cluster of chromosome 11p13 translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988 Jul;7(7):2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Font M. P., Loiseau P., Bories J. C., Degos L., Lefranc M. P., Sigaux F. The human T-cell V gamma gene locus: cloning of new segments and study of V gamma rearrangements in neoplastic T and B cells. Blood. 1988 Aug;72(2):776–783. [PubMed] [Google Scholar]
- Chen Z., Le Paslier D., Dausset J., Degos L., Flandrin G., Cohen D., Sigaux F. Human T cell gamma genes are frequently rearranged in B-lineage acute lymphoblastic leukemias but not in chronic B cell proliferations. J Exp Med. 1987 Apr 1;165(4):1000–1015. doi: 10.1084/jem.165.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dariavach P., Lefranc M. P. First genomic sequence of the human T-cell receptor delta 2 gene (TRDV2). Nucleic Acids Res. 1989 Jun 26;17(12):4880–4880. doi: 10.1093/nar/17.12.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Font M. P., Chen Z., Bories J. C., Duparc N., Loiseau P., Degos L., Cann H., Cohen D., Dausset J., Sigaux F. The V gamma locus of the human T cell receptor gamma gene. Repertoire polymorphism of the first variable gene segment subgroup. J Exp Med. 1988 Oct 1;168(4):1383–1394. doi: 10.1084/jem.168.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A., Huck S., Ghanem N., Lefranc M. P., Rabbitts T. H. New subgroups in the human T cell rearranging V gamma gene locus. EMBO J. 1987 Jul;6(7):1945–1950. doi: 10.1002/j.1460-2075.1987.tb02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Hagge T. E., Yokota S., Bartram C. R. Detection of minimal residual disease in acute lymphoblastic leukemia by in vitro amplification of rearranged T-cell receptor delta chain sequences. Blood. 1989 Oct;74(5):1762–1767. [PubMed] [Google Scholar]
- Hara J., Benedict S. H., Champagne E., Takihara Y., Mak T. W., Minden M., Gelfand E. W. T cell receptor delta gene rearrangements in acute lymphoblastic leukemia. J Clin Invest. 1988 Dec;82(6):1974–1982. doi: 10.1172/JCI113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Brenner M. B., Krangel M. S. Identification of putative human T cell receptor delta complementary DNA clones. Science. 1987 Oct 30;238(4827):678–682. doi: 10.1126/science.3499667. [DOI] [PubMed] [Google Scholar]
- Hata S., Clabby M., Devlin P., Spits H., De Vries J. E., Krangel M. S. Diversity and organization of human T cell receptor delta variable gene segments. J Exp Med. 1989 Jan 1;169(1):41–57. doi: 10.1084/jem.169.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Satyanarayana K., Devlin P., Band H., McLean J., Strominger J. L., Brenner M. B., Krangel M. S. Extensive junctional diversity of rearranged human T cell receptor delta genes. Science. 1988 Jun 10;240(4858):1541–1544. doi: 10.1126/science.3259726. [DOI] [PubMed] [Google Scholar]
- Hockett R. D., de Villartay J. P., Pollock K., Poplack D. G., Cohen D. I., Korsmeyer S. J. Human T-cell antigen receptor (TCR) delta-chain locus and elements responsible for its deletion are within the TCR alpha-chain locus. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9694–9698. doi: 10.1073/pnas.85.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S., Dariavach P., Lefranc M. P. Variable region genes in the human T-cell rearranging gamma (TRG) locus: V-J junction and homology with the mouse genes. EMBO J. 1988 Mar;7(3):719–726. doi: 10.1002/j.1460-2075.1988.tb02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S., Lefranc M. P. Rearrangements to the JP1, JP and JP2 segments in the human T-cell rearranging gamma gene (TRG gamma) locus. FEBS Lett. 1987 Nov 30;224(2):291–296. doi: 10.1016/0014-5793(87)80472-6. [DOI] [PubMed] [Google Scholar]
- Isobe M., Russo G., Haluska F. G., Croce C. M. Cloning of the gene encoding the delta subunit of the human T-cell receptor reveals its physical organization within the alpha-subunit locus and its involvement in chromosome translocations in T-cell malignancy. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3933–3937. doi: 10.1073/pnas.85.11.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J. Antigen receptor genes as molecular markers of lymphoid neoplasms. J Clin Invest. 1987 May;79(5):1291–1295. doi: 10.1172/JCI112951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Urban J. L., Hood L. In individual T cells one productive alpha rearrangement does not appear to block rearrangement at the second allele. J Exp Med. 1989 Dec 1;170(6):2183–2188. doi: 10.1084/jem.170.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Le Paslier D., Chen Z., Loiseau P., Cohen D., Sigaux F. T cell rearranging gene gamma: diversity and mRNA expression in fresh cells from T cell acute lymphoblastic leukemia. Blood. 1987 Sep;70(3):637–646. [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Forster A., Rabbitts T. H. Rearrangement of two distinct T-cell gamma-chain variable-region genes in human DNA. 1986 Jan 30-Feb 5Nature. 319(6052):420–422. doi: 10.1038/319420a0. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gellert M. The mechanism of antigen receptor gene assembly. Cell. 1989 Nov 17;59(4):585–588. doi: 10.1016/0092-8674(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Loiseau P., Guglielmi P., Le Paslier D., MacIntyre E., Gessain A., Bories J. C., Flandrin G., Chen Z., Sigaux F. Rearrangements of the T cell receptor delta gene in T acute lymphoblastic leukemia cells are distinct from those occurring in B lineage acute lymphoblastic leukemia and preferentially involve one V delta gene segment. J Immunol. 1989 May 1;142(9):3305–3311. [PubMed] [Google Scholar]
- Macintyre E., d'Auriol L., Amesland F., Loiseau P., Chen Z., Boumsell L., Galibert F., Sigaux F. Analysis of junctional diversity in the preferential V delta 1-J delta 1 rearrangement of fresh T-acute lymphoblastic leukemia cells by in vitro gene amplification and direct sequencing. Blood. 1989 Nov 1;74(6):2053–2061. [PubMed] [Google Scholar]
- Quertermous T., Strauss W. M., Van Dongen J. J., Seidman J. G. Human T cell gamma chain joining regions and T cell development. J Immunol. 1987 Apr 15;138(8):2687–2690. [PubMed] [Google Scholar]
- Quertermous T., Strauss W., Murre C., Dialynas D. P., Strominger J. L., Seidman J. G. Human T-cell gamma genes contain N segments and have marked junctional variability. Nature. 1986 Jul 10;322(6075):184–187. doi: 10.1038/322184a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Satyanarayana K., Hata S., Devlin P., Roncarolo M. G., De Vries J. E., Spits H., Strominger J. L., Krangel M. S. Genomic organization of the human T-cell antigen-receptor alpha/delta locus. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8166–8170. doi: 10.1073/pnas.85.21.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takihara Y., Tkachuk D., Michalopoulos E., Champagne E., Reimann J., Minden M., Mak T. W. Sequence and organization of the diversity, joining, and constant region genes of the human T-cell delta-chain locus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6097–6101. doi: 10.1073/pnas.85.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. J., Poplack D. G., Bakhshi A., Reaman G., Cole D., Jensen J. P., Korsmeyer S. J. Gene rearrangements as markers of clonal variation and minimal residual disease in acute lymphoblastic leukemia. J Clin Oncol. 1987 May;5(5):735–741. doi: 10.1200/JCO.1987.5.5.735. [DOI] [PubMed] [Google Scholar]
- Yamada M., Hudson S., Tournay O., Bittenbender S., Shane S. S., Lange B., Tsujimoto Y., Caton A. J., Rovera G. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5123–5127. doi: 10.1073/pnas.86.13.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]