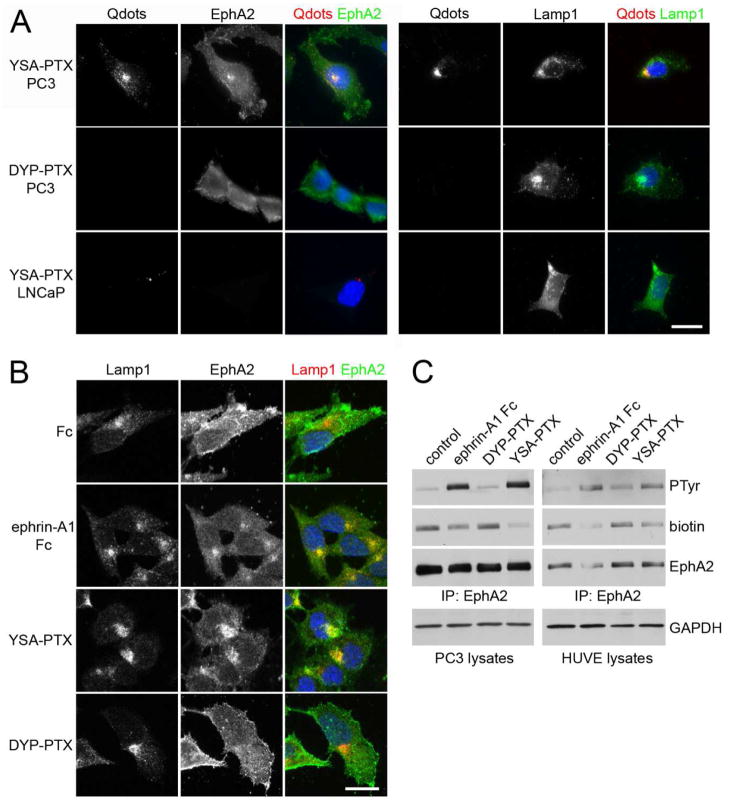
Figure 4. YSA-PTX is internalized in cancer and endothelial cells expressing EphA2.
(A) YSA-PTX coupled to fluorescent quantum dots, but not DYP-PTX, is internalized with EphA2 into lysosomes of prostate cancer cells expressing EphA2. PC3 prostate cancer cells, which express high levels of EphA2, or LNCaP prostate cancer cells, which do not detectably express EphA2 protein, were treated for 20 min with 100 μM YSA-PTX or control DYP-PTX, followed by a 20 min incubation with streptavidin-conjugated quantum dots (Qdots). After removing the solution containing the peptides and the quantum dots, the cells were incubated for 2 h at 37°C to allow EphA2 internalization induced by YSA-PTX binding. The cells were stained for EphA2 (left) or the lysosomal marker Lamp1 (right). Qdots were also imaged and nuclei were labelled with DAPI in blue. Representative fluorescence micrographs are shown. Scale bar = 25 μM. (B) Ephrin-A1 Fc and YSA-PTX, but not DYP-PTX, causes EphA2 internalization into lysosomes. PC3 cells were treated for 2 h with 0.2 μg/mL ephrin-A1 Fc, 100 μM YSA-PTX or 100 μM control DYP-PTX. The cells were stained for Lamp1 (red) and EphA2 (green). Nuclei were labelled with DAPI (blue). Representative confocal micrographs are shown. Scale bar = 25 μm. (C) Ephrin-A1 Fc and YSA-PTX, but not DYP-PTX, cause EphA2 internalization into cells. PC3 prostate cancer and HUVE endothelial cells were treated for 1 h with 0.2 μg/mL ephrin-A1 Fc, 100 μM YSA-PTX or 100 μM control DYP-PTX. Proteins present on the cell surface were then labeled with biotin. EphA2 immunoprecipitates were probed with anti-phosphotyrosine antibody (PTyr), streptavidin-HRP (biotin) and EphA2 antibody. The amount of GAPDH in the cell lysates used for the immune-precipitations is also shown as a control for equal amounts of protein in the lysates used for immunoprecipitation.