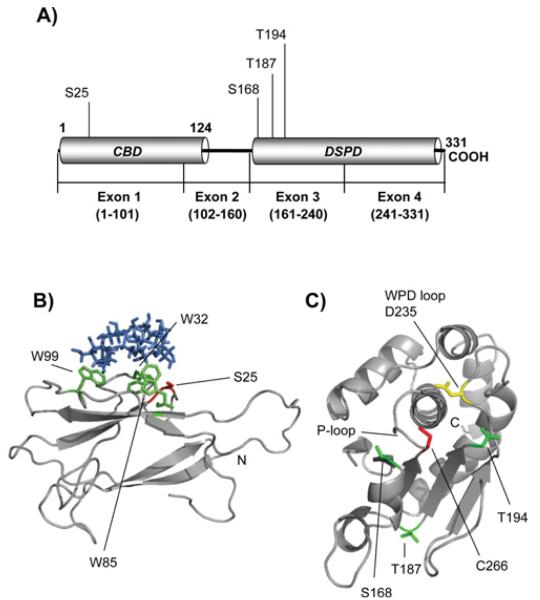
Fig. 3.
Structure models of laforin CBD and DSPD domains. A) Diagram of the position of the CBD and DSPD domain in the primary structure of human laforin. The positions of residues described in this work are also indicated. B) Laforin CBD domain (residues 1-116) was in silico modelled, as described in Experimental section, using the crystal structure of Geobacillus stearothermophilus cyclodextrin glycosyltransferase (PDB: 1CYG) as a template. The positions of the Trp residues involved in carbohydrate binding (W32, W85 and W99) are indicated in green and the position of residue Ser25 is marked in red. β-cyclodextrin is coloured in blue. The N-terminus is also indicated. C) The laforin dual specificity phosphatase domain (residues 157-326) was in silico modelled, as described in Experimental procedures, using the crystal structure of human DUSP22 (PDB: 1WRM) as a template. The position of the characteristic P-loop, containing the catalytic C266 residue (in red), and the WPD-loop, containing the conserved D235 (in yellow), is indicated. The positions of other residues described in this study (S168, T187 and T194; in green) and the C-terminus are also indicated.