Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) induce gastric injury on long-term usage. This study aims at reducing the side effect of NSAIDs by encapsulating in zein, an acid-resistant biopolymer. Aceclofenac-loaded zein microspheres were prepared by emulsification and solvent evaporation method. The stability of zein microspheres at gastric pH retarded the release of the entrapped drug and hence reduces the possibility of gastric injury. However, the in vitro release of aceclofenac was sustained up to 72 h at intestinal pH. Thus, zein microspheres pave the way for the development of safe and sustained delivery system for NSAIDs thereby achieving the desired therapeutic potential with reduced side effects for chronic inflammatory disorders.
Key words: biopolymers, drug delivery, gastric injury, NSAIDs, zein microspheres
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most popular and only competent drugs prescribed for a large number of connective tissue disorders. Osteoarthritis and rheumatoid arthritis are the most predominant connective tissue disorders ranging about approximately 100 million patients being treated annually [1]. Majority of these disorders require chronic usage of NSAIDs resulting in a high risk of gastrointestinal toxicity and injury [2]. The only possible management includes a prophylactic therapy with a proton pump inhibitors to reduce symptoms. NSAID treatment has been an absolute requirement for patients with connective tissue disorder requiring pain relief. Therefore, alternative drug delivery systems with less or no side effects were screened for a safer therapy. Microencapsulation is one of the techniques used to alter the dosing frequency by sustaining the release of the entrapped drug and minimizing the unwanted effects [3]. The hitherto known biopolymers such as alginate or gelatin cannot be used as a carrier for oral delivery of NSAIDs, owing to their instability at acidic pH. We therefore screened zein, a major storage protein of corn belonging to a class of prolamins, for the preparation of microspheres of NSAIDs. These proteins are made up of two microheterogeneous groupings with molecular weights of 23.8 and 26.7 kDa [4]. The solubility characteristics of zein are restricted to solvents like ethanol, acetone, or acetonyl acetone. This is attributed to its high content of hydrophobic non-polar amino acids [5]. Zein forms tough, glossy hydrophobic coatings with antibacterial activity and has been of importance in food industry [6]. Zein has been used to develop films composed of microspheres with good biocompatibility [7]. The ability of zein to deliver various classes of drugs and proteins is well documented [8–12]. The behavior of zein microspheres in the presence and absence of enzymes has been found in the literature [13]. The hydrophobicity, reduced susceptibility to proteolytic degradation, and its ability to withstand gastric pH make it suitable for oral as well per oral delivery of drugs for chronic disorders.
Aceclofenac is a nonsteroidal anti-inflammatory drug used extensively in the treatment of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. Aceclofenac is one of the emerging NSAID molecules [14]. The short biological half-life (<4 h) and dosing frequency of more than one time a day make it an ideal candidate for modified release formulation. In this study, the preparation of aceclofenac-loaded zein microspheres was investigated. The effect of drug/zein ratio, extent of glutaraldehyde cross-linking on the percentage of drug entrapped, release characteristics from the developed zein microspheres, and its biocompatibility were studied. These microspheres were then loaded in capsules to develop a convenient dosage form for the formulation and characterized the drug release pattern.
MATERIALS AND METHODS
Materials
Biochemically pure zein was obtained from Sigma Life Sciences (Sigma–Aldrich, St. Louis, USA). Aceclofenac was kindly obtained as a gift sample from Ind-swift Ltd. Chandigarh, India, and all other reagents were of reagent grade.
Preparation of Aceclofenac-Loaded Zein Microspheres
Aceclofenac-loaded zein microspheres (Acl-Zn-Ms) were prepared by modified emulsification and solvent evaporation method [15]. The spheres were prepared by dissolving the drug in polymer which was previously dissolved in 90% alcohol. This solution was added to the continuous phase (sesame oil) containing 0.5% span 80 as an emulsifying agent, and the mixture was then agitated using a homogenizer with the rotating speed of 400 rpm. Then, toluene saturated with glutaraldehyde (0.25, 0.5, and 1 ml) was added as a cross-linking agent. The dispersed drug and zein were immediately transformed into fine droplets, which subsequently solidified into rigid microspheres due to solvent evaporation. The particles were collected by filtration, washed with petroleum ether, and desiccated under vacuum for 24 h.
Formulation of Capsules Containing Aceclofenac-Loaded Zein Microspheres
The Acl-Zn-Ms cross-linked with 1 ml of toluene saturated with glutaraldehyde were measured directly and manually placed into a hard gelatin capsule of size 0. The microspheres were maintained at a drug dose of 20 mg (300 mg of Acl-Zn-Ms) per capsule. Dissolution measurement was performed on these capsules.
Morphology Analysis
The morphology of the zein microspheres was observed using a scanning electron microscope (SEM, Hitachi). The microspheres were vacuum dried, mounted onto brass stubs, and sputter coated with gold in argon atmosphere prior to observation under SEM. The mean diameter and particle size distribution of the microspheres dispersed in water were measured using a laser light-scattering particle size analyzer (Microtrac S3500).
Compatibility Between the Components of Drug-Loaded Microspheres
Fourier transformed infrared (FTIR) spectra of aceclofenac, zein, and Acl-Zn-Ms were obtained using ABB instrument by mixing samples with potassium bromide, compressed into a pellet by applying 1 ton/unit. Spectral scanning was done in the range of 4,000–400 cm−1.
Physical State of the Components in the Drug-Loaded Microspheres
Differential scanning calorimetry (DSC) of drug, polymer, and Acl-Zn-Ms cross-linked with three different concentrations was obtained using TA instruments, Q-200. The samples were hermetically sealed in aluminum pans and heated at a constant rate of 10°C/min over a temperature range of 25–250°C. To maintain an inert atmosphere, nitrogen gas was purged at a rate of 20 ml/min.
Drug Loading and Encapsulation Efficiency
The amount of aceclofenac entrapped within the microspheres was determined by dissolving the microspheres in 90% ethanol and analyzed using an UV spectrophotometer (Perkin-Elmer, USA) at 273 nm. Drug loading and encapsulation efficiency were determined using the following equations:
 |
In Vitro Release of Aceclofenac from Zein Microspheres
The in vitro release studies were performed at three different pH values: (1) pH 1.2, i.e., simulated gastric fluid pH and (2) pH 6.8 and 7.4, i.e., simulated intestinal fluid pH. Dissolution was carried out on the microspheres at 37°C at 100 rpm.
A weighed quantity of Acl-Zn-Ms (100 mg) was suspended in 100 ml of dissolution media consisting of 0.1 N hydrochloric acid, and the dissolution was done for 2 h. At the end of 2 h, disodium hydrogen phosphate and potassium dihydrogen phosphate were added to make the pH 6.8; the dissolution was continued for 4 h. After the total 6 h, the pH of the medium was changed to 7.4 by adding disodium hydrogen phosphate and sodium chloride, and the release study was continued. The sample was withdrawn at regular intervals and replaced with the same volume of test medium. The withdrawn samples were diluted suitably and spectrophotometrically estimated for aceclofenac at 273 nm.
In Vitro Release of Aceclofenac from Capsules Filled with Acl-Zn-Ms
Capsules containing Acl-Zn-Ms (300 mg) and cross-linked with 1 ml of toluene saturated with glutaraldehyde were placed in 100 ml of dissolution medium, and the release study was carried out similarly as described above.
Cytotoxicity Studies
The biocompatibility of the cross-linked microspheres was evaluated by performing MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) assay [16] using fibroblast cells from explant tissue. Cells were seeded in 12-well plates (7,000 cells/well) containing Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum, allowed to adhere, and maintained for 24 h. Then the medium was replaced with DMEM containing 1% serum. Uncross-linked Acl-Zn-Ms, 0.2 mg, and cross-linked Acl-Zn-Ms with 0.25 ml and 1 ml of toluene saturated with glutaraldehyde were added. The test samples were kept in triplicate. Controls were maintained under similar conditions without the influence of microspheres. The plate was incubated at 37°C in 5% CO2. After 72 h, the cell proliferation was measured spectrophotometrically at 570 nm. The procedure involved the addition of MTT stock solution (5 mg/ml) to each culture well, equal to one tenth of the original culture volume, and incubated at 37°C for 3–4 h. At the end of the incubation period, the medium was removed, and the purple formazan crystals formed were solubilized with dimethyl sulfoxide. Absorbance of the converted dye was measured at a wavelength of 570 nm.
Statistical Analysis
Experiments were repeated three times, and the results were expressed as mean and standard deviation/standard error from the three replicates.
RESULTS
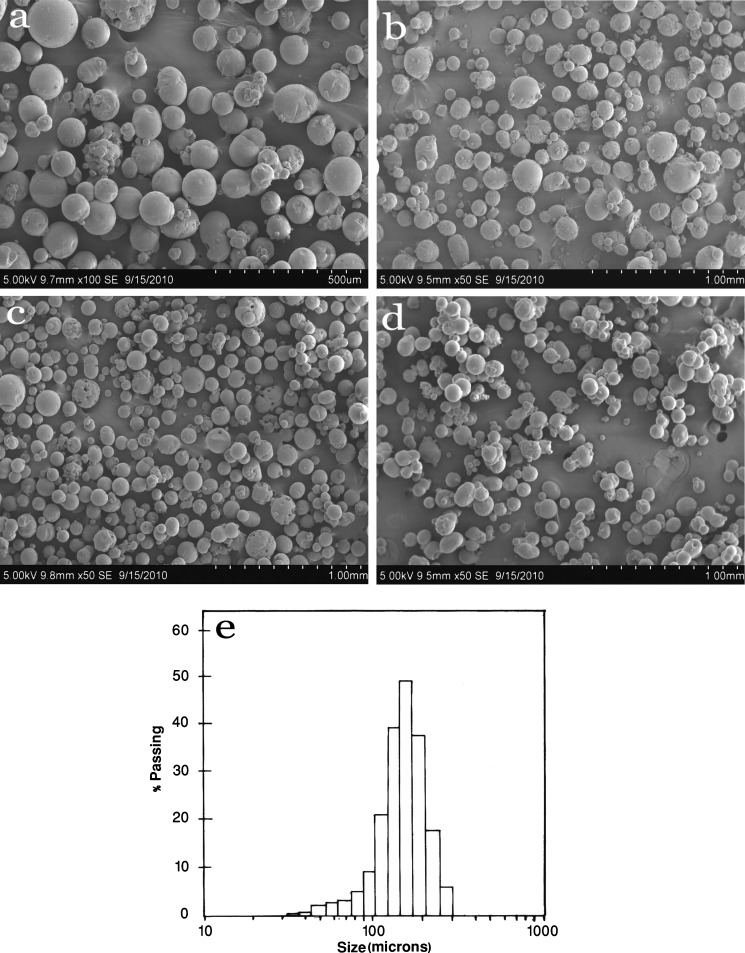
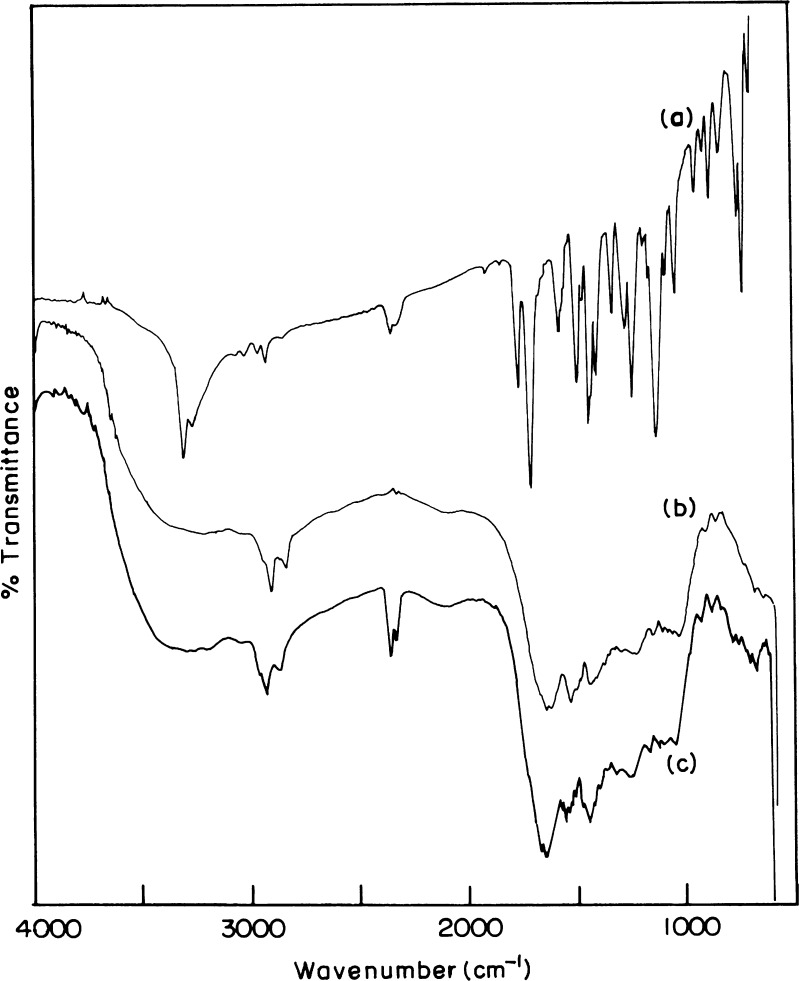
In the present study, Acl-Zn-Ms were prepared with various drug–polymer ratio and different cross-linker concentrations. The SEM of blank and cross-linked microspheres (0.25, 0.5, and 1 ml) was shown in Fig. 1 (a, b, c, and d). The surface of the developed microspheres was porous in nature. Particle size analysis (Fig. 1e) showed that the microspheres were heterogeneous in size ranging between 30 and 300 μm. The average particle size of uncross-linked microspheres and 0.25, 0.5, and 1 ml cross-linked microspheres were found as 174 ± 34.66, 147 ± 29.14, 143 ± 35.65 and 136 ± 25.92 μm, respectively. The FTIR spectrum of aceclofenac (Fig. 2a) showed characteristic C=O stretching at 1,772 and 3318 cm−1, carboxylic group absorption band at 1,717 cm−1, N–H stretching at 1,589 cm−1, O–H bending at around 1,055 cm−1, and C=C stretching at 2,362 cm−1. The FTIR spectrum of zein (Fig. 2b) shows characteristic peaks at around 1,645 cm−1, 1,541 cm−1, and 1,236 cm−1 corresponding to amide I, II, and III, respectively. Zein bands with only a minor contribution of the aceclofenac bands dominated the FTIR spectrum of Acl-Zn-Ms (Fig. 2c). This spectrum showed the peaks corresponding to COOH and C=C stretching of the drug at 1,717 and 2,362 cm−1, respectively.
Fig. 1.
Scanning electron micrographs of zein microspheres. a Uncross-linked, b cross-linked with 0.25, c 0.5, and d 1 ml of toluene saturated with glutaraldehyde. e Particle size distribution of zein microspheres
Fig. 2.
FTIR spectrum of a aceclofenac, b zein, and c aceclofenac-loaded zein microspheres
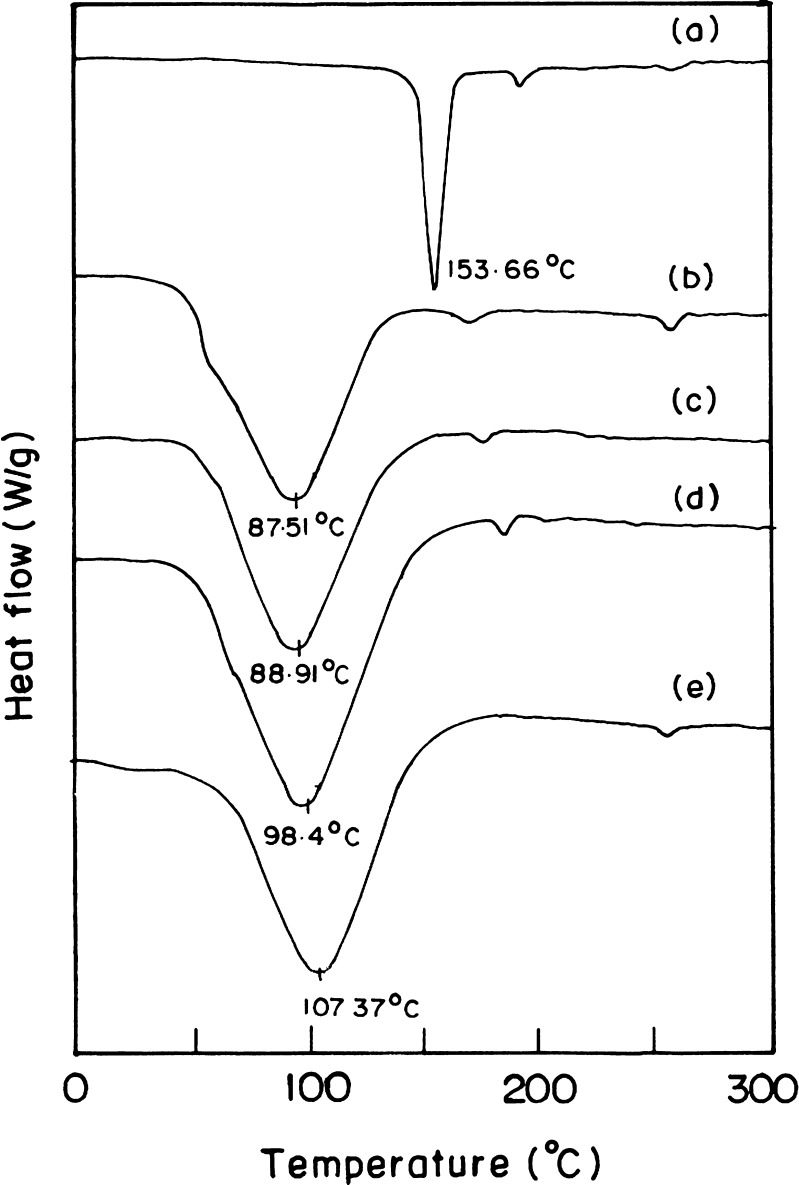
The DSC thermograms of aceclofenac, zein, and Acl-Zn-Ms uncross-linked with 0.25, 0.5, and 1 ml were depicted in Fig. 3. The endothermic peak of zein was observed at 87°C, and the melting temperature of aceclofenac was found as 153°C. Acl-Zn-Ms cross-linked with 0.25, 0.5, and 1 ml of toluene saturated with glutaraldehyde showed endothermic peaks at 89°C, 98°C, and 107°C, respectively. Aceclofenac loading and encapsulation efficiency were found to be dependent on the concentration and ratio of aceclofenac to zein (Table I).
Fig. 3.
DSC thermograms of a aceclofenac, b zein, c aceclofenac-loaded zein microspheres cross-linked with 0.25, d 0.5, and e 1 ml of toluene saturated with glutaraldehyde
Table I.
Aceclofenac loading and encapsulation efficiency in zein microspheres
| Formulation code | Drug/polymer (mg) | Concentration of cross-linker (ml) | Drug loading (% w/w)a | Encapsulation efficiency (% w/w)b |
|---|---|---|---|---|
| F 1 | 100:500 | 0 | 4.92 ± 0.05 | 24.5 ± 0.38 |
| F 2 | 100:300 | 5.05 ± 0.07 | 15.15 ± 0.30 | |
| F 3 | 250:500 | 8.63 ± 0.05 | 14.38 ± 0.23 | |
| F 4 | 100:500 | 0.25 | 4.74 ± 0.21 | 21.34 ± 0.94 |
| F 5 | 100:300 | 4.77 ± 0.40 | 12.87 ± 1.08 | |
| F 6 | 250:500 | 8.70 ± 0.54 | 26.11 ± 1.62 | |
| F 7 | 100:500 | 0.5 | 5.25 ± 0.34 | 23.58 ± 1.53 |
| F 8 | 100:300 | 4.28 ± 0.37 | 11.56 ± 1.01 | |
| F 9 | 250:500 | 6.67 ± 0.55 | 20.02 ± 1.66 | |
| F 10 | 100:500 | 1 | 4.79 ± 0.45 | 21.54 ± 2.06 |
| F 11 | 100:300 | 4.38 ± 0.66 | 11.82 ± 1.77 | |
| F 12 | 250:500 | 6.85 ± 0.56 | 20.53 ± 1.68 |
aDrug loading (percent weight/weight) = amount of drug in microspheres/amount of microspheres taken
bEncapsulation efficiency (percent weight/weight) = amount of drug in microspheres/drug initially added
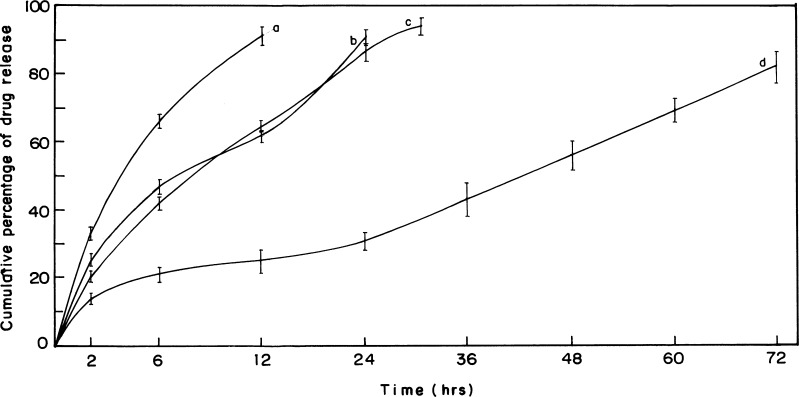
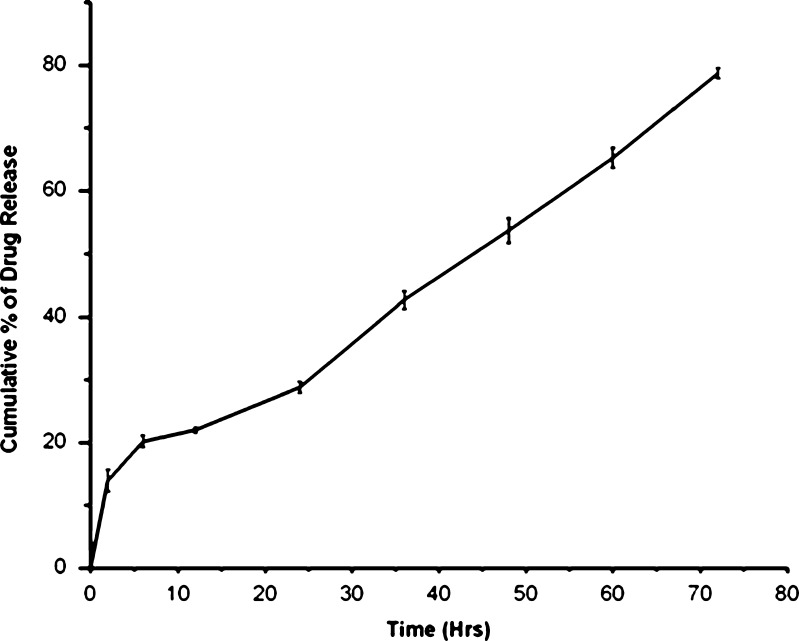
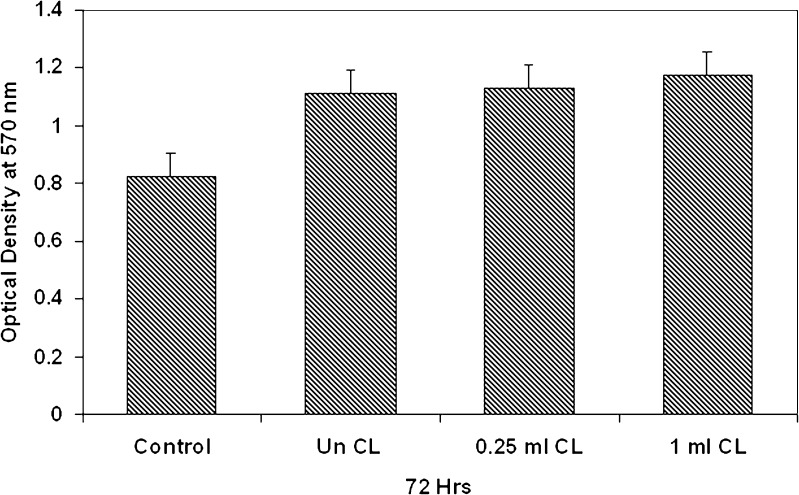
The release curves (Fig. 4) indicated a sustained release pattern following zero order kinetics. It appeared biphasic and might be attributed to the permeability and hydrophobic nature of zein. The uncross-linked Acl-Zn-Ms showed a sustained release up to 12 h (Fig. 4a), and almost 90% of drug release was achieved. In Acl-Zn-Ms cross-linked with 0.25 ml of toluene saturated with glutaraldehyde, the release was extended up to 24 h (Fig. 4b). The microspheres cross-linked with 0.5 ml exhibited an enhanced sustainability extending up to 30 h (Fig. 4c). At the end of 72 h, more than 85% of entrapped drug was released from the formulation cross-linked with 1 ml of cross-linker (Fig. 4d). The release profile of aceclofenac from zein microspheres (cross-linked with 1 ml of toluene saturated with glutaraldehyde) which filled the hard gelatin capsules was given in Fig. 5. Only 14% of the drug was released during the first 2 h of the study at pH 1.2, and almost 79% of the drug was released at the end of 72 h. Biocompatibility of the zein microspheres was evaluated in vitro using fibroblast cells from explant tissue. No significant differences were observed between uncross-linked and cross-linked groups (Fig. 6).
Fig. 4.
In vitro release profiles from aceclofenac-loaded zein microspheres. a Uncross-linked, cross-linked with b 0.25, c 0.5, and d 1 ml of toluene saturated with glutaraldehyde. (Data were the mean ± SD of three measurements.)
Fig. 5.
In vitro release profile of hard gelatin capsules containing aceclofenac-loaded zein microsphere. (Data were the mean ± SD of three measurements.)
Fig. 6.
Cytotoxicity assay of aceclofenac-loaded zein microspheres cross-linked with toluene saturated with glutaraldehyde at 72 h
DISCUSSION
The application of natural polymers and proteins such as gelatin, albumin, and casein has been in vogue for quite some time. However, owing to their rapid solubilization in aqueous environments, the sustainable drug release patterns have not been achieved. Further chemical cross-linking with glutaraldehyde and formaldehyde enabled fast release to be overcome, but the presence of residual cross-linking agents induces toxic side effects. Therefore, hydrophobic polymeric system such as zein was chosen and used as encapsulation matrix. Zein has additional advantages of low toxicity and absorbability of the degraded end products. The emulsification and solvent evaporation method, being simple and easy to scale up compared to other methods [17], have been employed for the preparation of zein microspheres. Further, the problems encountered owing to the residual effects of glutaraldehyde were overcome by the use of toluene saturated with glutaraldehyde. The cytotoxicity study confirmed the non-toxic nature of the developed microspheres. The in vitro release studies indicated the ability of zein to withstand the acidic pH and retard the release of entrapped substance. This property is therefore suitable for the development of oral drug delivery systems affecting the gastric mucosa. These unique features of zein such as biocompatibility, acid resistant ability, and sustainability make it a promising material for the development of novel drug delivery systems. Moreover, capsules are considered as oral delivery vehicles for microspheres owing to their convenience in development along with damage minimization during fabrication in comparison to the tabletted microspheres. The hard gelatin capsules enable the retention of shape and integrity of the microspheres. Thus, capsules may be considered as a robust oral delivery vehicle for zein microspheres.
The Acl-Zn-Ms were found to be spherical, free flowing, and discrete without any aggregation. Moreover, cross-linking did not induce any alteration in the surface morphology although the mean diameter was slightly reduced as observed in the SEM images and particle size analyzer. The FTIR spectrum of Acl-Zn-Ms was biased towards the absorption bands of lower energy because of the wavelength-dependent penetration depth of the IR radiation [18]. Only the drug molecules adsorbed on the surface of the spheres were detected and resulted in limited peaks of aceclofenac in the spectrum. Hence, no chemical interaction between the drug and polymer was found. DSC studies were performed to understand the nature and interaction of the encapsulated drug in the matrix. The absence of aceclofenac peak in the DSC could be attributed to the existence of the drug in an amorphous or disordered crystalline phase as a molecular dispersion in polymer matrix [19]. An increase in the concentration of the cross-linker enhanced the physiochemical characteristics of the zein microspheres but did not modify the drug loading and encapsulation efficiency. Drug loading increased with an increase in the aceclofenac concentration while encapsulation efficiency was enhanced with an increase in the zein concentration. The formulations F3, F6, F9, and F12 exhibited optimum drug loading efficiency and hence were selected for in vitro release studies and biocompatibility analysis. In an attempt to use zein microspheres as a potential delivery system for NSAIDs, we have examined the in vitro release profiles of aceclofenac from uncross-linked and cross-linked zein microspheres. In simulated gastric fluid (pH 1.2), all the formulations showed minimum release (less than 20%). However, changing the pH from 1.2 to 6.8, a burst effect was observed owing to the drug entrapped on the surface of the microspheres. Further increasing the pH to 7.4, a slow drug release at a constant rate was observed. The sustained release of the entrapped drug could be attributed to the hydrophobicity of zein on one hand while the cross-linking retarded the penetration of the medium into spheres on the other hand. Thus, by altering the concentration of the cross-linking agent, the desired release profile of the delivery system could be achieved. In capsules, the reduction in the release rate may be due to the decrease in the wettability of the microspheres during the initial hours of release study. Later, the release profile of the capsules followed the similar pattern of zein microspheres. Moreover, at simulated gastric pH, the drug released from zein microspheres was minimal. This property of zein makes it suitable for the development of delivery systems for NSAIDs so as to minimize the adverse ulcerative side effects. The maximum in vitro release of the drug was observed at 72 h, and hence, cytotoxicity studies were carried out up to 72 h. The increase in concentration of the cross-linking agent did not induce any toxicity even at 72 h, and it was encouraging to know that zein microspheres enhanced the cell proliferation (Fig. 6). This may be attributed to the additional elemental source supplied by the amino acid moieties of zein. Further, zein microspheres might have provided better surface area for adhesion as well as binding of cells [7] and hence enhanced the proliferation.
CONCLUSION
The in vitro evaluation of the zein microspheres exhibits the uniqueness of the formulation. This microcarrier system is not only attributed with the desired pain relief but also significantly retards the gastric disturbances induced by NSAIDs. Moreover, the biocompatible and hydrophobic nature of zein microspheres makes it robust for the development of scaffolds composed of microspheres for tissue regeneration. This type of delivery system based on zein opens up new avenues in the design of safer therapeutic modalities.
ACKNOWLEDGMENTS
The first author is extremely grateful to the Council of Scientific and Industrial Research, New Delhi, for the research fellowship. We are grateful to Dr. A.B. Mandal, Director, Central Leather Research Institute, for providing the encouragement and facilities to carry out this work.
REFERENCES
- 1.James MS. The impact of non-steroidal anti-inflammatory drug-induced gastropathy. Am J Manag Care. 2001;7:10–14. [PubMed] [Google Scholar]
- 2.Doherty M, Hunt RH, Langman MJS, Pounder RE, Russell RI, Sturrock RD, Thould AK. Management of NSAID induced gastrointestinal disturbance. Ann Rheum Dis. 1987;46:640–643. doi: 10.1136/ard.46.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obeidat WM, Price JC. Preparations and evaluation of Eudragit S100 microspheres as pH sensitive release preparation for piroxicam and theophylline using the emulsion solvent evaporation method. J Microencap. 2006;23:195–202. doi: 10.1080/02652040500435337. [DOI] [PubMed] [Google Scholar]
- 4.Hamaker BR, Mohamed AA, Habben JE, Huang CP, Larkens BA. Efficient procedure for extracting maize and sorghum kernel proteins reveals higher prolamin contents than the conventional method. Cereal Chem. 1995;72:583–588. [Google Scholar]
- 5.Gennadios A, Weller CL. Edible films and coatings from wheat and corn proteins. Food Technol. 1990;44:63–69. [Google Scholar]
- 6.Shukla R, Munir C. Zein: the industrial protein from corn. Ind Crop Prod. 2001;13:171–192. doi: 10.1016/S0926-6690(00)00064-9. [DOI] [Google Scholar]
- 7.Dong J, Sun Q, Wang JY. Basic study of corn protein, zein as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials. 2004;25:4691–4697. doi: 10.1016/j.biomaterials.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein H, Morrel E, Mathiowitz E, Schwaller K, Thomas R. Protein microspheres and methods of using them. US Patent No. 5, 679, 377, 1999.
- 9.Hurtado L, Murdan S. Formulation and characterization of zein microspheres as delivery vehicles. J Drug Deliv Sci Tec. 2005;15:267–272. [Google Scholar]
- 10.Liu XM, Sun QS, Wang HJ, Zhang L, Wang JY. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials. 2006;51:39–43. doi: 10.1016/j.biomaterials.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Muthuselvi L, Aruna D. Simple coacervates of zein to encapsulate gitoxin. Colloid Surface. 2006;51B:39–43. doi: 10.1016/j.colsurfb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Wang HJ, Lin ZX, Liu XM, Sheng SY, Wang JY. Heparin-loaded zein microspheres film and hemocompatibility. J Control Release. 2005;105:120–131. doi: 10.1016/j.jconrel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Hurtado L, Murdan S. Zein microspheres as drug/antigen carriers: a study of their degradation and erosion in the presence and absence of enzymes. J Microencap. 2006;23:303–314. doi: 10.1080/02652040500444149. [DOI] [PubMed] [Google Scholar]
- 14.Parfitt K. Analgesic anti-inflammatory and anti-pyretic In: Reynolds JE, editor. Martindale, the complete drug reference, 32nd ed. Massachusetts; 1999. p. 2–12.
- 15.Mathiowitz E, Bernstein H, Morrel E, Schwaller K. Method for producing protein microspheres. WO Patent No. 91/06286, 1991.
- 16.Mosmann T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm. 2008;363:26–39. doi: 10.1016/j.ijpharm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Weert MVD, Hof RV, Weerd JVD, Heeren RMA, Posthuma G, Hennink WE, et al. Lysozyme distribution and conformation in a biodegradable polymer matrix as determined by FTIR techniques. J Control Release. 2000;68:31–40. doi: 10.1016/S0168-3659(00)00227-3. [DOI] [PubMed] [Google Scholar]
- 19.Corrigan OI. Thermal analysis of spray dried products. Thermochim Acta. 1995;248:245–258. doi: 10.1016/0040-6031(94)01891-J. [DOI] [Google Scholar]