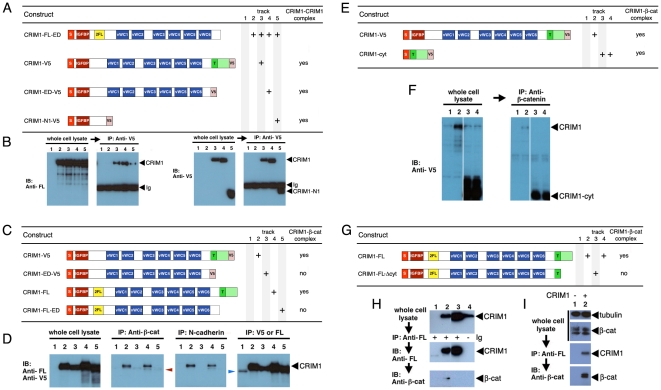
Figure 7. CRIM1 self-associates via the N-terminal domain and forms a complex with ß-catenin and N-cadherin via the C-terminal domain.
(A, C, E, G) Tables showing CRIM1 expression constructs used in pull-down assays and result summaries. (B) According to anti-V5 immunoprecipitations from the same whole-cell lysates, CRIM1-V5 (track 3), CRIM1-ED-V5 (track 4) and CRIM1-N1-V5 (track 5) all interact with CRIM1-FL-ED suggesting multimerization via the N-terminal region represented in CRIM1-N1-V5. The immunoglobulin used for immunoprecipitation is detected in the immunoblot of (B), right panels of each pair (Ig). (D) Anti-V5 and anti-FLAG (FL) immunoblots of whole-cell lysates show all CRIM constructs shown in (C) express to abundant levels in 293 cells. Control anti-V5, anti-FLAG immunoprecipitation followed by anti-V5, anti-FLAG immunoblot shows that CRIM1 proteins are readily detected. Immunoblots of the same immunoprecipitations with anti-ß-catenin or anti-N-cadherin antibodies show that CRIM1 forms complexes with ß-catenin and N-cadherin but only if the CRIM1 cytoplasmic domain is present. The band in track 1 of D (blue arrowhead) is a background band. (F) Anti-V5 immunoblots of whole cell lysates (left panel) and anti-ß-catenin immunoprecipitations (right panel) show a version of CRIM1 with the signal peptide, trans-membrane domain and cytoplasmic domain forms a complex with ß-catenin. (H) immunoprecipitation using anti-FLAG antibodies in 293 cells expressing either CRIM1-FL or CRIM1-FLΔcyt. ß-catenin co-immunoprecipitates with CRIM1-FL, track 2, but not with CRIM1-FLΔcyt, track 3. Track 4 shows a control IP from 293 cells expressing CRIM1-FL where no primary antibody was added. No ß-catenin association is observed. (I) Co-immunoprecipitation of ß-catenin with FLAG-tagged CRIM1-FL with 293 whole cell lysate shows detection of endogenous ß-catenin. Anti-tubulin western blot was shown as loading control. In all experiments, an equal proportion of lysate is represented on compared gel tracks.