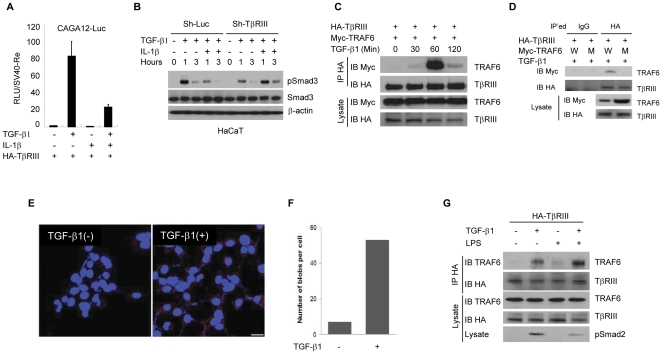
Figure 3. TRAF6 forms a complex with TβRIII in response to TGF-β1.
(A) CAGA12-Luciferase assays were performed in HepG2 cells. The plasmids encoding HA-TβRIII, TRAF6, CAGA12-Luc, and Renilla-luc reporter gene were transfected as indicated and, on the next day, TGF-β1 (0.4 ng/ml) and/or IL-1β (20 ng/ml) was added for 16 hours. The obtained relative luciferase units(RLU) were normalized by renilla luciferase activities. (B) Using the control and TβRIII knock-down HaCaT cells, TGF-β1 (0.4 ng/ml) and/or IL-1β (20 ng/ml) were treated as shown for up to 3 hours. The level of total Smad3 and phospho-Smad3 protein was detected by immunoblotting. For the control of equal loading, β-actin was used. (C) HEK293 cells stably expressing HA-TβRIII were transfected with Myc-Traf6 plasmids and then treated with TGF-β1. Cells were harvested at various times and were subjected to immunoprecipitation with anti-HA antibody. Co-immunoprecipitated TRAF6 was detected with anti Myc antibody. (D) Complex formation ability between TβRIII and TRAF6 wild-type or the TRAF6 (C85A/H87A) E3 ligase mutant was compared after TGF-β stimulation for an hour. (E) According to the manufacturer's protocol, interaction was visualized by in situ proximity ligation assay (O-link) with proximity probes directed against TRAF6 and TβRIII using Alexa 555 labeling (red). Endogenous TRAF6 was co-localized with HA-TβRIII along the plasma membrane in the presence of TGF-β (red blobs). Bar = 2.5 µm. (F) Quantification of blobs per cell was carried out by semi-automated image analysis using the freeware software BlobFinder V 3.0. (G) HA-TβRIII-stably expressing 4T07 mouse mammary cancer cells were treated with TGF-β and LPS for one hour. Co-immunoprecipitation was carried out using anti-HA antibody to query interaction with endogenous TRAF6. The results are representative of three independent experiments.