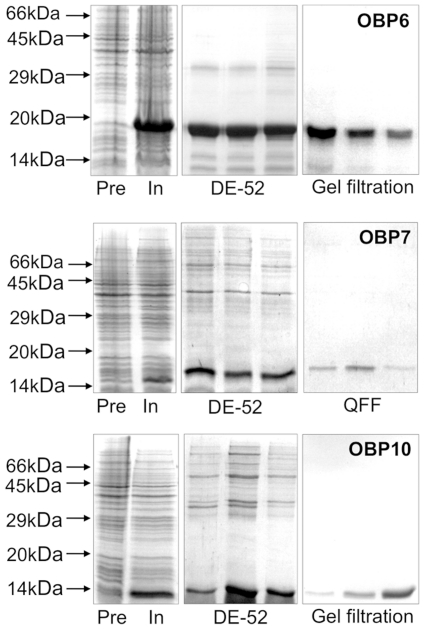
Figure 2. Expression and purification of A. pisum OBP6, OBP7 and OBP10.
Proteins were expressed in BL21(DE3)pLysS E. coli cells transformed with pET-30b vector containing the appropriate OBP sequence. Recombinant proteins were obtained in high yields (about 20 mg/L) as inclusion bodies and were solubilised by denaturation and refolding as described in the Materials and Methods sections. Purification was accomplished by combinations of chromatographic steps on anion-exchange resins, such as DE-52 (Whatman) and QFF (GE-Healthcare), followed by gel filtration on Sephacryl-100 or Superose-12 (GE-Healthcare) along with standard protocols previously adopted for other odorant-binding proteins.