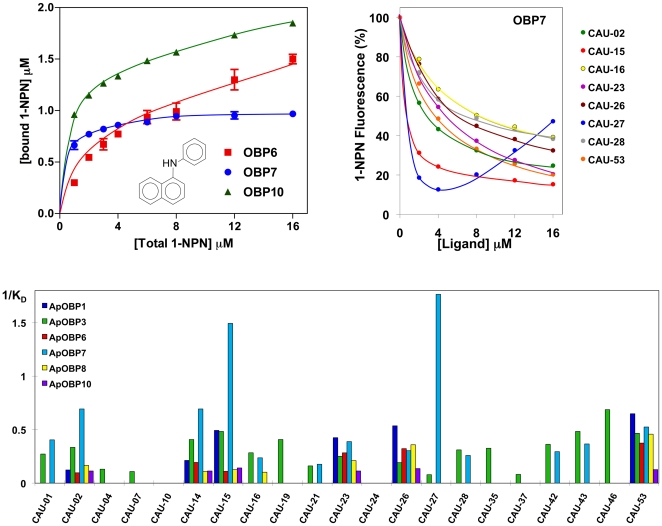
Figure 4. Binding of CAU ligands to the six A. pisum OBPs.
Upper left panel. Binding of 1-NPN to the recombinant OBP6, OBP7 and OBP10. 2 µM solutions of each protein in 50 mM Tris-HCl, pH 7.4 was titrated with 1 mM solution of 1-NPN in methanol to final concentrations of 2–16 µM. The data, averages of three replicates, were analysed using Prism software and indicate in each protein the presence of a single binding site. Dissociation constants were 6.3 (SEM 1.0), 0.54 (SEM 0.05) and 1.2 (SEM 0.15) for OBPs 6, 7 and 10, respectively. The same software showed that saturation occurs at one binding site/monomer for OBP6 and OBP7, but at one binding site/dimer in the case of OBP7. Upper right panel. Competition binding assays of selected CAU ligands to OBP7. In each experiment a mixture of the protein and 1-NPN in Tris, both at the concentration of 2 µM, was titrated with 1 mM methanol solutions of the competing ligands to final concentrations of 2–16 µM. Fluorescence intensities are reported as percent of the values in the absence of competitor. Lower panel. Reverse values of the dissociation constants measured with all 21 CAU ligands and the six A. pisum OBPs. The values of the dissociation constants are reported as supplementary information in Table S1. The structures of the 21 CAU ligands are reported in Table S3.