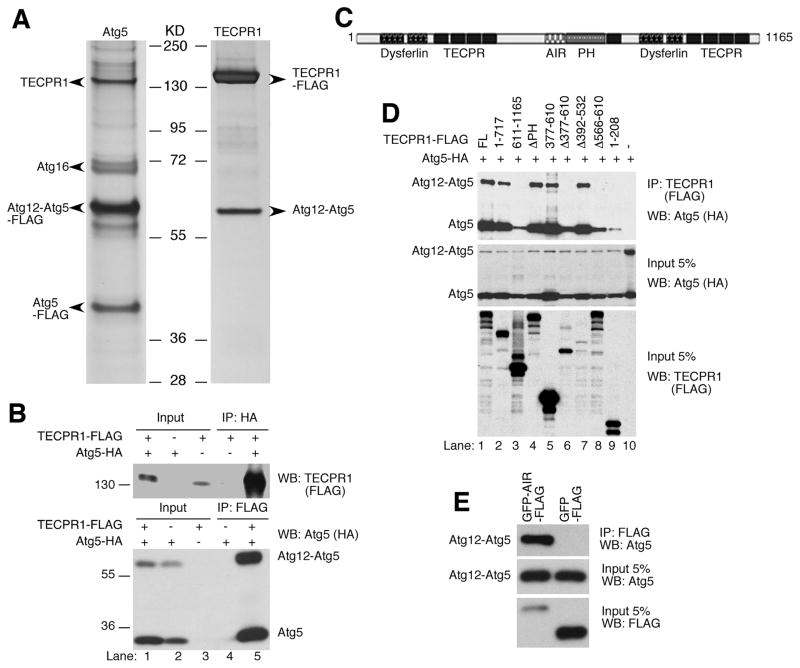
Figure 1. TECPR1 interacts with the conjugated Atg12-Atg5.
(A) Silver staining of the tandem affinity-purified Atg5 complexes or TECPR1 complexes in HEK293T cells. Proteins noted on the gel were identified by mass spectrometry. (B) Reciprocal co-immunoprecipitation of TECPR1 and Atg12-Atg5. HEK293T cells were transfected with TECPR1-FLAG and Atg5-HA. Whole cell lysate was immunoprecipitated with anti-FLAG or anti-HA agarose and analyzed by Western Blotting. (C) Domain structure of TECPR1. TECPR1 contains a pleckstrin homology (PH, aa611–717) domain, an Atg12-Atg5 interacting region (AIR, aa566–610), nine beta-propeller repeats (TECPR, aa209–240, 254–285, 301–332, 344–376, 729–756, 953–984, 1044–1075, 1087–1127), and two dysferlin domains (aa64–170, 816–922). (D) AA566–610 of TECPR1 is essential for Atg12-Atg5 binding. Atg5-HA was coexpressed with TECPR1-FLAG or different mutants as indicated. Whole cell lysate was immunoprecipitated with anti-FLAG agarose, followed by immunoblotting with anti-HA antibody. (E) The AIR domain of TECPR1 is sufficient for Atg12-Atg5 binding. Whole cell lysate was immunoprecipitated with anti-FLAG agarose. See also Figure S1.