Abstract
Background
Cholestasis is a common disease of the liver. Chronic cholestasis eventually leads to hepatic cirrhosis and fibrosis, and rodent chronic cholestasis models are used to study aspects of fibrosis and cirrhosis. Cholestasis-induced liver injury and fibrosis are associated with increased oxidative stress and inflammation. Few pharmacological therapies exist for treatment of cholestasis or cirrhosis, but it is known that humans with better nutritional intake are less likely to develop certain types of cirrhosis. Eugenia jambolana (Jamun) is a tropical berry fruit rich in antioxidant anthocyanin compounds.
Aim
Because anthocyanins decrease cellular lipid peroxidation and oxidative stress, it was hypothesized that Jamun fruit extract (JFE) administration could protect against cholestatic liver injury and inflammation in mice.
Method
Starting 24 h after sham or bile-duct ligation (BDL) surgery, male C57Bl/6 mice were administered vehicle or JFE (100 mg/kg, po) for ten days.
Results
Mice that underwent BDL had elevated serum ALT levels, which were reduced 60% by JFE treatment. Likewise, BDL caused hepatic inflammation, macrophage infiltration, fibrosis, and necrosis, all of which were largely improved by JFE. Interestingly, hepatoprotection was observed in JFE-treated BDL mice, despite suppressed transporter expression and increased hepatic bile acid concentrations.
Conclusion
Jamun fruit phytochemicals decreased hepatic inflammation and oxidative stress, and protected against hepatocellular injury in mice. Jamun warrants further investigation as a potential antioxidant/anti-inflammatory therapy to treat not only cholestasis, but also other liver diseases with an inflammatory component.
Keywords: cholestasis, inflammation, polyphenol, cirrhosis, NF-κB, Eugenia jambolana
Introduction
Eugenia jambolana (Jamun) is a berry fruit, which grows abundantly in tropical regions of South Asia and South America, and is used in traditional medicines such as Ayurveda (1). Both Jamun fruit and seed extracts possess antioxidant activities (2, 3). Jamun contains polyphenolic anthocyanin derivatives, such as delphinidin-3,5-diglucoside and petunidin-3,5-diglucoside (4), that inhibit cyclooxygenase activity, an enzyme that plays a key role in inflammation (5). Moreover, delphinidin-3,5-diglucoside have been previously shown to decrease lipid peroxidation and thiobarbituric acid reactive substances (TBARS) in naïve rat liver and serum (6, 7).
Cholestasis is a pathological condition caused by obstruction of hepatic bile flow (8). Impaired biliary excretion increases hepatic bile acid concentrations and stimulates production of reactive oxygen species – both events have been implicated in the subsequent hepatocyte necrosis and apoptosis (8–10). In response to BDL-induced hepatocellular necrosis, kupffer cells, and neutrophils are activated and recruited to sites of damage (11). These immune cells secrete pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, by stimulating the transcription factor Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KappaB; NF-κB) signaling pathway (9, 12, 13). By 2 to 3 weeks after bile-duct ligation, fibrosis can be seen within the liver (9). Therapeutic options for treating cholestasis in humans are limited and therefore, research is needed to identify novel modalities. Additionally, certain aspects of cholestasis as a disease are similar to other hepatic diseases such as alcoholic cirrhosis, non-alchoholic steatosis, non-alcoholic cirrhosis, and hepatitis – particularly inflammation. Therefore, therapies that target shared mechanisms of injury may improve not only cholestatic disease, but also other hepatic pathologies.
It has long been thought that bile acid accumulation and inflammation in liver are key components to hepatocellular damage during cholestasis. This is supported in part by the adaptive regulation of several transporters involved in bile acid enterohepatic circulation in response to BDL (14). The liver appears to coordinately down-regulate expression of bile acid uptake transporters and enhance levels of efflux transporters in an attempt to prevent intracellular accumulation of bile acids (15). Therefore, regulation of bile acid transporters may be an additional mechanism to exploit pharmacologically in developing therapies to treat cholestatic injury.
Because multiple pathways contribute to the progression of liver injury, the purpose of this study was to evaluate whether treatment of bile-duct ligated mice with JFE could 1) reduce the extent and severity of liver necrosis 2) suppress inflammation and lipid peroxidation, 3) alter compensatory expression of hepatobiliary transporters, and 4) prevent hepatic accumulation of bile acids. Characterization of the whole Jamun fruit extract was performed to evaluate potential in vivo activity before testing individual components. Collectively, these studies tested the utility of Jamun for treating cholestatic liver damage in mice and demonstrate that JFE does afford partial protection against cholestatic liver injury, fibrosis, and inflammation.
Materials and Methods
Jamun Fruit Extract (JFE) Preparation
JFE was prepared from freeze-dried Jamun fruit pulp using a methanol extraction method as described by Li et al 2009 (4, 16). The JFE was enriched for anthocyanin content. Briefly, freeze-dried whole fruit powder was sequentially and exhaustively extracted with cold hexanes, followed by ethyl acetate, and then acidified methanol (0.1% hydrochloric acid). The extract obtained was reconstituted in water and enriched for anthocyanin content by using adsorption chromatography on an XAD-16 Amberlite resin column. Water (5 L) was added to the column to elute sugars and acids, and followed with acidic methanol (0.1% hydrochloric acid) to afford a red-purple JFE. The anthocyanin content was determined by using the pH differential method and was calculated as equivalents of cyanidin-3-glucoside, using the extinction coefficient of 26900 L cm−1 mg−1 and a molecular mass of 449.2 g/L (4, 16). Individual anthocyanins were identified by high performance liquid chromatography with ultraviolet (HPLC-UV) and tandem mass spectrometry (LC-MS/MS) methods (16). JFE contained 3.5% anthocyanins (as cyanidin-3-glucoside equivalents) which occur as diglucosides of five anthocyanidins/aglycons: delphinidin, cyanidin, petunidin, peonidin and malvidin (4, 16).
Bile Duct Ligation Surgery and JFE Administration
Sham or BDL surgery was performed on adult male C57Bl/6 mice purchased from Jackson Laboratories (Bar Habor, ME) under pentobarbital-induced anesthesia (65 mg/kg, i.p). Body temperature was maintained throughout the surgery using rectal thermometers calibrated to 37°C attached to thermal lamps (Braintree Scientific, Braintree, MA). Beginning 24 hours after surgery, mice were dosed daily with either JFE suspended in 0.5% methylcellulose and 0.2% tween 80 (100 mg/kg, 10ml/kg) or vehicle (10ml/kg) by oral gavage for ten days. The dose selected was based on a previous study, which demonstrated Jamun protection against acetaminophen-induced liver toxicity in rats (17). Blood and livers were collected 24 hours following the last JFE treatment after euthanasia under isoflurane-induced anesthesia. Tissues were snap frozen in liquid nitrogen and stored at −80°C. Serum was isolated from blood by centrifugation at 5000 rpm for 5 min at 4°C and stored at −80°C. All animal experiments were performed at the University of Rhode Island, College of Pharmacy animal facility with IACUC approval. Numbers of mice used per treatment group were decided by use of power analysis in accordance with institutional IACUC recommendations.
RNA Isolation
Total RNA was isolated from liver tissue by phenol-chloroform extraction, using RNA Bee reagent (TelTest Inc., Friendswood, TX) according to the manufacturer’s protocol. RNA concentration was quantified by measuring absorbance at 260 nm using a UV spectrometer (Nanodrop ND 1000, Nanodrop Inc.), and all samples were diluted to 1μg/μL. RNA integrity was confirmed by formaldehyde-agarose gel electrophoresis.
Hematoxylin and Eosin Staining
A portion of the central lobe of the liver was fixed in formalin for 24 hour, transferred to 75% ethanol, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin (AML Laboratories, Rosedale, MD). A board-certified veterinary pathologist evaluated liver sections by light microscopy for histopathological changes.
Hepatic Fibrosis Quantification
Hepatic fibrosis was quantified using Masson trichrome staining and the hydroxyproline assay. Hepatic hydroxyproline levels were quantified according to the manufacturer’s protocol using a colorimetric hydroxyproline assay kit (Biovision, Mountview, CA). Liver tissue (50 mg) was homogenized in 500μl of dH2O. Homogenate (100μl) was incubated with 100μl of 12N HCl at 120°C for 3 hours. Hydrolyzed sample (10μl) was transferred to a 96-well plate and allowed to dry. Chloramine T (100μl) was added to each well and incubated at 60°C for 90 min and absorbance was read at 560nm.
Measurement of Alanine Aminotransferase (ALT) and Bile Acid Concentrations
Serum ALT levels were quantified using an Infinity™ kit according to the manufacturer’s protocol (Thermo Scientific, Middletown, VA). Bile acids were extracted from liver using a t-butanol extraction method as described previously (18). Serum and liver bile acids levels were measured using a spectrophotometric bile acid assay kit (Bioquant, San Diego, CA).
Quantification of Serum and Liver Cytokine Levels
Circulating and tissue-specific cytokines were quantified using luminex-based assays that analyze multiple targets in a single assay. Serum Interleukin (IL) -1α, 1β, 6, 10, Monocyte chemotactic protein-1 (Mcp-1, Ccl2), and Tumor necrosis factor α (Tnf-α) were quantified using a mouse cytokine 6-plex assay kit according to the manufacturer’s protocol (Bio Rad®, Hercules, CA, USA). Hepatic Tnf, IL-1β, IL-3, IL-4, IL-8 receptor β (Cxcr2), Ccl2, Tumor growth factor β (Tgfβ), Interferon-gamma-induced protein (Cxcl-10, Ip-10) mRNA levels were measured with a mouse cytokine plex assay kit (Affymetrix®, Fremont, California). All multiplex assays were performed with a Bio-plex 200 system (Bio-Rad®, Hercules, CA, USA).
Oligonucleotide Probesets for Branched DNA (bDNA) Assay
Probe sets for mouse Mrp2, Bsep, Oatp1a1, 1a4, 1b2, Ntcp, Ost α, β, Nrf2 and Nqo1, have been previously described (19–22). Mouse gene sequences of interest were acquired from GenBank. Multiple oligonucleotide probe sets (capture extender, CE, label extender, LE, and blocker, BL probes) were designed using ProbeDesigner software version 1.0 (Bayer Corp., Emeryville, CA) to be highly specific to a single mRNA transcript. All oligonucleotide probes were designed with a 63°C melting temperature. Each probe designed in Probe Designer was submitted to National Center for Biotechnological information nucleotide comparison by the basic local alignment search tool (BLASTn) to ensure minimum cross reactivity with other mouse sequences. Oligonucleotides with a high degree of similarity to other mouse gene transcripts were eliminated from the design. Oligonucleotide probesets required for the assay were graciously donated by Dr. Curtis Klaassen (University of Kansas Medical Center, Kansas City, KS).
bDNA Amplification Assay
All reagents for analysis including lysis buffer, amplifier/label probe diluent, and substrate solution were supplied in the Quantigene 1.0 Reagent System (Panomics, Fremont, CA). Oligonucleotide probes were diluted in lysis buffer. 10μl RNA (1μg/μl) samples were added to each well of the 96-well plate containing 50μl of capture hybridization buffer and 100μl of diluted probe set. RNA was allowed to hybridize overnight with probe set at 53°C. After overnight incubation, subsequent hybridization steps were followed as mentioned in the manufacturer’s protocol, and luminescence was measured with a GloRunner™ microplate luminometer interfaced with GloRunner DXL Software (Turner Biosystems, Sunnywale, CA). Luminescence was recorded as relative light units (RLU) per 10μg of total RNA.
Real-time Quantitative PCR
Liver-specific cytokine-like inter-cellular adhesion molecule 1 (Icam-1), keratinocyte-derived chemokine (KC, Cxcl-1), macrophage inflammatory protein 2α (Mip-2α, Cxcl-2), and inducible nitric oxide synthase (iNos) mRNA levels were quantified using quantitative PCR. cDNA was obtained by reverse transcribing 1μg total RNA using Superscript IIH Reverse Transcriptase (Roche, Germany). Real-time quantitative PCR was performed using Power SYBRH Green PCR Master Mix (SA BioSciences, Frederick, MA) with a Light Cycler 480 II (Roche, Germany) and Light Cycler® 480 software Release 1.5.0, version1.5.0.39 for analysis. Sequences for primers are listed in the supplementary information (Table i).
Liver Membrane and Cytosolic Preparations
Liver membrane and cytosol fractions were obtained by homogenization with a dounce homogenizer using 150mM sucrose/10mM tris-HCl buffer along with differential ultracentrifugation (100,000 × g for 1 h). Protein concentrations were determined using a Dc Protein assay (Bio-Rad, Hercules, CA, USA).
Western Blots
Western blots were performed to quantify relative Ntcp and Bsep protein expression in liver. Ntcp (K4) and Bsep (K44) antibodies were generously donated by Bruno Steiger (University Hospital, Zurich, Switzerland). Membrane preparations (50μg protein/well) were solubilized in Laemmli buffer and β-mercaptoethanol and electrophoretically separated on a 10% tris-polyacrylamide gel at 150V for 60 min and transferred onto a PVDF membrane at 90V for 50 min. The membrane was blocked with 5% Non fat dry milk (NFDM) in tris-buffered saline with 0.05% Tween 20 (TBS/T) for 1 hr. After blocking, the membrane was incubated overnight with appropriate concentrations of primary antibodies diluted in 5%NFDM in TBS/T at 4°C and subsequently with corresponding horseradish peroxidase labeled secondary antibodies also diluted in 5%NFDM in TBS/T for 1hr at room temperature. Blots were then incubated in ECL-Plus western blot detection reagent and exposed on X-ray films. The blots were quantified using Quantity-one® Basic (version 4.6.9) software (Bio-Rad Laboratories). Ntcp and Bsep protein quantification of Ntcp and Bsep performed is presented in supplementary data.
Thiobarbituric Acid Reactive Substance (TBARS) Assays
Liver malondialdehyde concentration was quantified using a commercially available kit (Oxi-Tek TBARS assay kit, Zeptometrix Corp., Buffalo, NY). Livers were homogenized in phosphate-buffered saline and protein concentrations determined by a modified Lowry assay using the Biorad Dc protein assay (Bio-Rad Laboratories, Hercules, CA). Sodium dodecyl sulfate and TBARS diluent were added to samples and incubated at 95 °C for 60 min. After incubation, samples were cooled to 4°C and absorbance was measured at 530nm. Amount of MDA levels were normalized to total protein concentration.
Macrophage Staining
Frozen liver tissue sections (5 μm) were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 min at room temperature, washed once in PBS, and once in PBS with 0.2% Triton-X 100. Fixed sections were incubated at room temperature for 1 hr with 5% goat serum in 0.2% PBS with Triton-X 100 (PBS-T), and then 2 hours with F4/80 (Serotec, Raleigh, NC) and CD68 (Abcam, Cambridge, MA) antibodies diluted to 1:100 and 1:800 respectively in 5% goat serum/PBS-T. Next, slides were washed with PBS-T for 5 min and then incubated for 1 hr at room temperature in goat anti-rat Alexa Fluor® 488 IgG secondary antibody (1:100 and 1:850 respectively in 5% goat serum/PBST) and phalloidin conjugated to Alexa Fluor® 568 (1:200) (Invitrogen, Eugene, OR) diluted in PBS-T. Slides were washed with PBS-T for 5 min, washed with deionized distilled water, and air dried. Slides were mounted with Prolong Gold with DAPI and stored at −20°C until they were imaged with a Nikon Eclipse E600 microscope (Nikon Instruments Inc., Melville, NY) and a SPOT RT camera (Diagnostic instruments, Inc.).
NF-kB Binding Activity Assay
Nuclear proteins were extracted from pooled liver tissues using a TransAm nuclear protein extraction kit (Active Motif, Carlsbad, CA). The concentration of the pooled nuclear protein samples was determined by the Bradford assay (Bio-Rad®, Hercules, CA) according to the manufacturer’s protocol. NF-kB binding activity (20μg nuclear protein per well) was measured using a TransAm NF-kB (p-65 subunit) assay kit (Active Motif, Carlsbad, CA) according to manufacturer’s protocol. The assay was performed three times and the data represent the average optical density (OD) at 450nm.
Media Nitrite Concentrations
RAW 264.7 cells derived from murine macrophages were obtained from the American Type Culture Collection (Manassas, VA). RAW 264.7 cells were plated at a density of 1 × 105 cells/well in a 96-well plate with 100μl of culture medium and incubated at 37°C for 24 h. Cells were treated with JFE (1, 10, 25, 50, 75, 100, 125, 150 ppm) and stimulated with LPS(Sigma Cat:L3755, LPS from E.coli 026:b6) (10ng/ml) for 24 h at 37°C. After incubation, nitrite concentrations in media were measured 15 min after adding modified Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in distilled water) (Sigma-Aldrich, St. Louis, MO). Optical density at 540 nm was measured using a Spectramax microplate reader (Sunnyvale, CA). Nitrite concentrations were compared to standard solutions of sodium nitrite prepared in culture medium.
Statistical Analysis
The statistical significance between groups was determined by factorial ANOVA followed by a Duncan’s Multiple-range post hoc test, using Statistica 9.1 software (StatSoft, Inc., Tulsa, OK). Data are presented as mean± SE, with p ≤0.05 considered statistically significant.
Results
JFE decreases BDL-induced liver injury
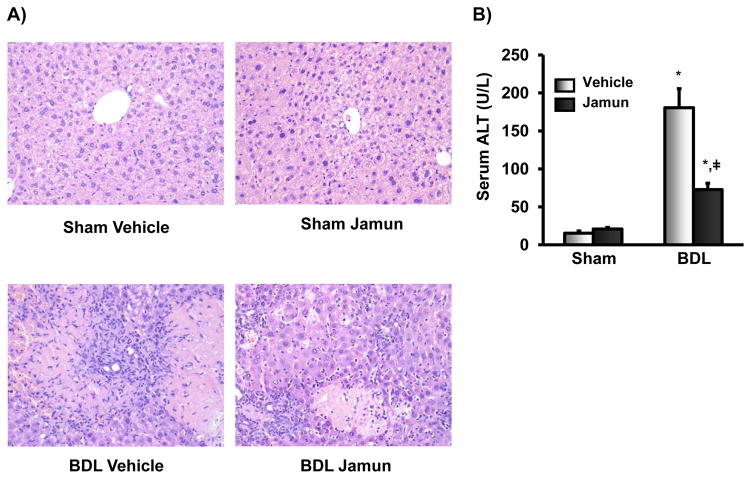
Figure 1 illustrates the effect of vehicle or JFE treatment on BDL-induced hepatocyte damage. Histopathologic evaluation of livers from BDL mice demonstrated variable hepatocellular necrosis, biliary hyperplasia, hepatocellular regeneration, and leukocyte infiltration that was slightly less severe in JFE-treated mice. These data indicate that JFE reduces injury of hepatocytes in mice induced by BDL (Fig. 1A). BDL increased serum ALT activity, an indicator of hepatocellular injury, by 10-fold compared to mice that underwent sham surgery. Treatment of BDL mice with JFE significantly decreased serum ALT levels by 60% (Fig. 1B).
Figure 1.
Markers of liver injury in mice after sham or bile-duct ligation (BDL) surgery and concomitant treatment with vehicle or Jamun. A) Hematoxylin and Eosin staining for mice livers. Piece of liver tissue were fixed in formalin prior to routine processing and paraffin embedding. Sections (5μm) of liver were stained with hematoxylin and eosin and examined by light microscopy for hepatocellular degeneration and necrosis, inflammation, and bile duct proliferation. (B) Serum ALT levels were quantified using colorimetric assay. Data are presented as mean ALT levels (U/L) ± S.E (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05).
JFE decreases markers of BDL-induced fibrosis
Trichrome staining demonstrated that BDL increased collagen deposition in liver, as anticipated (Fig. 2A). JFE treatment decreased collagen staining (Fig. 2A). Compared to vehicle treatment, BDL significantly increased hydroxyproline levels, which was prevented by JFE treatment (Fig. 2B). JFE had no effect on serum or hepatic bile acids in mice that underwent sham surgery (Fig. 2C). BDL significantly increased serum and liver bile acid concentrations. In mice that underwent BDL and JFE treatment tended to decrease bile acid levels in serum as compared to vehicle-treated mice, although not statistically significant (Fig. 2C, upper panel). BDL increased bile acid concentrations in livers of vehicle-treated mice by 1.8-fold, and unexpectedly elevated levels to a greater extent in JFE-treated mice (Fig. 2C, lower panel) although there is decrease in fibrosis.
Figure 2.
Markers of cholestatic injury in mice after sham or bile-duct ligation (BDL) surgery and concomitant treatment with vehicle or JFE. A) Masson Trichome staining for visualizing hepatic collagen staining in BDL mice. Hepatic fibrosis was attenuated with JFE treatment in BDL mice. All image were taken at 200X magnification. (B) Liver hydroxyproline level, a marker for tissue fibrosis levels was determined. Data presented as mean hydroxyproline levels (μg/μl/g of tissue) ± S.E.M (n=4–5 mice per group). (C) Bile acid concentrations were quantified in serum and liver. Data presented as mean bile acid levels (μmole/10μl of serum and μmole/100mg of tissue) ± S.E.M (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05).
JFE suppresses hepatic transporter expression
Induction and manipulation of hepatic transporter expression can be protective against cholestastic injury, especially in the BDL model (8, 15, 23). Therefore, the ability of JFE to induce transporters that transport bile acids and bilirubin was evaluated (Figs. 3, 4, and supplementary data Figure-i). In sham-operated mice, JFE decreased Oatp1a4 and 1b2 mRNA by 76 and 35%, respectively (Fig. 3A). Similarly, BDL decreased Ntcp, Oatp1a1, and Oatp1b2 expression, irrespective of treatment, by at least 46, 93, and 44% of shams, respectively. In BDL mice, JFE treatment decreased Ntcp mRNA to levels below mice that received vehicle. JFE decreased Oatp1b2 mRNA expression in sham and BDL mice by 35 and 41% respectively, compared to vehicle-treated sham and BDL mice. BDL increased Oatp1a4 mRNA in livers of both treatment groups by approximately 2-fold, as compared to shams. JFE decreased Oatp1a4 mRNA expression by 76 and 44% in sham and BDL mice, respectively, as compared to vehicle-treated mice. BDL significantly decreased Ntcp protein levels in both vehicle-and JFE-treated mice compared to shams (Fig. 3B).
Figure 3.
Hepatic uptake transporters expression involved in bile acid uptake. (A) Sodium-dependent taurocholate co-transporting polypeptide (Ntcp), Organic anion transporting polypeptide (Oatp) 1a1,1a4 and 1b2 mRNA expression. mRNA was quantified using branched DNA signal amplification assay. Data presented as mean relative light unit (RLU) per 10μg total RNA ± S.E.M (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05). (B) Bile acid uptake transporter Ntcp and housekeeping gene Gapdh protein levels were determined using western blot.
Figure 4.
Hepatic canalicular transporters expression involved in bile acid efflux. (A) Multidrug resistance-associated protein 2 (Mrp2), bile salt export pump (Bsep), Organic solute transporter (Ost) α and β mRNA expression. mRNA was quantified using branched DNA signal amplification assay. Data presented as average relative light unit (RLU) per 10μg total RNA ± S.E.M (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05). (B) Bile acid efflux transporter Bsep and housekeeping gene Gapdh protein levels were determined using western blot.
In vehicle-treated mice, BDL upregulated Ostα/β mRNA expression about 3 fold (Fig. 4A). JFE prevented induction of Ost-α and β mRNA expression after BDL surgery. In sham-operated mice, JFE treatment decreased liver Mrp2 mRNA by 59% compared to vehicle controls (Fig. 4A). BDL significantly increased Mdr2 expression in liver in both treatment groups by at least 2-fold. Mrp2 and Bsep mRNA expression were not altered by BDL alone, but were lower in mice that received JFE after BDL. Similar to mRNA results, BDL did not alter Bsep protein levels in liver. JFE treatment did not affect Bsep protein expression (Fig. 4B).
JFE reduces pro-inflammatory cytokine and chemokine expression
BDL can cause hepatic inflammation which involves increased pro- and anti-inflammatory cytokine expression (24). Similar to a published report (25), BDL increased IL-6, IL-1α, and Ccl2 concentrations in serum of vehicle-treated mice between 3- to 6-fold, as compared to sham mice (Fig. 5A). Compared to vehicle treatment, JFE reduced circulating levels of IL-6 by 35%, but had little effect on Ccl2 and IL-1α. Serum IL-10 levels were not altered significantly by BDL or JFE treatment (data not shown). In vehicle-treated mice, BDL increased mRNA expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF in liver by 17-, 3- and 10-fold as compared to sham mice (Fig. 5B). JFE treatment diminished IL-6 and IL-1β mRNA induction, although the IL-1β data were not statistically different. BDL significantly increased mRNA of anti-inflammatory cytokines, such as Cxcl10 and IL-10, in livers of vehicle-treated mice (3- and 1.5-fold, respectively), which was largely prevented by JFE. Similar to cytokines, mRNA expression of neutrophil – chemoattractant chemokines and adhesion molecules, such as Icam-1, Cxcl-1 and 2, were markedly increased by BDL (Fig. 6). JFE prevented mRNA induction of some chemokines and related genes (Icam-1 and Cxcl2), but not others (Cxcl1, Ccl2, Cxcr2). Neutrophil recruitment to the liver promotes hepatocellular injury after BDL and can be quantified by myeloperoxidase (MPO) activity (supplementary data Figure-iii) (26). JFE did not significantly decrease MPO activity in liver after BDL.
Figure 5.
Serum cytokine, interleukin (IL) 6, 1α, and monocyte chemotactic protein-1 (MCP-1, Ccl2) levels in mice. Cytokine protein levels was quantified using Plex assay. Data presented as average concentration (pg/ml) ± S.E.M (n=4–5 mice per group). B)Hepatic mRNA expression of IL 6,1β, 10,8rβ, Ccl2, Tumor necrosis factor (Tnf), Interferon inhibitory protein (Cxcl10, IP-10) and Transforming growth factor beta-2 (Tgfb-2). Cytokine mRNA expression was quantified using Quantigene 2.0 plex assay. Data are presented as average median fluorescent intensity (MFI) per 500ng total RNA ± S.E.M (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05).
Figure 6.
Effect of JFE on neutrophil accumlation in liver. A) Hepatic mRNA expression of inter-cellular adhesion molecule 1 (Icam-1), macrophage inflammatory protein 2(Cxcl2, Mip-2α) and keratinocyte-derived chemokine (KC, Cxcl1). Cytokine mRNA expression was quantified using RT-PCR. Data are presented as mean percent control of relative mRNA expression ± S.E.M (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05).
JEF decreases macrophage infiltration in liver after BDL
Kupffer cells are liver-specific macrophages that infiltrate regions of injury and can either promote or suppress inflammation (27). To assess whether JFE treatment have any effect on macrophage infiltration during cholestasis, immunofluorescent staining of macrophage biomarkers such as F4/80-positive and CD-68 was performed on frozen liver sections (Fig. 7A and 7B, and supplementary data Figure-iv). Liver cells are observed by staining actin cytoskeleton with phalloidin and nuclei. BDL increased macrophage staining, compared to respective sham mice. JFE significantly decreased macrophage infiltration in mice after BDL, although no apparent difference in zonal distribution of F4/80 staining was observed. Both F4/80 and CD-68 staining showed JFE treatment decrease in macrophage infiltration in BDL mice.
Figure 7.
Immunohistochemical staining of liver sections for macrophages. 5μm section were prepared form frozen tissues and fixed in 4% buffered formalin. Blocking with goat serum for 1 hour followed by 2 hours incubation with F4/80 and CD68 antibody and incubated with secondary antibody for 1 hour. These sections were also stained with rhodamine and prolong gold DAPI. All Images were taken at 200X magnification. In this tricolor staining green indicates macrophages, blue indicates nucleus and red indicates β-actin. A) Immunohistochemical staining for F4/80 antibody. B) Immunohistochemical staining for CD68 antibody.
JFE decreases reactive oxygen substances in liver after BDL
BDL increases bile acid concentrations in liver, which stimulate production of reactive oxygen species that can contribute to lipid peroxidation and hepatocellular damage. The process of lipid peroxidation produces malondialdehyde (MDA). MDA levels in vehicle- treated BDL mice increased 1.5 fold compared to shams whereas JFE treatment significantly decreased MDA levels by 25% in BDL mice (Fig. 8A). The expression of Nrf2, a transcription factor activated during oxidative stress and target gene, Nqo1, corresponded with liver MDA concentrations. Nrf2 and Nqo1 mRNA expression increased (by 2.2- and 3.3-fold respectively) in vehicle-treated BDL mice compared to shams (Fig. 8B). In BDL mice, JFE treatment significantly reduced Nrf2 and Nqo1 mRNA expression in liver by 67% and 58% respectively compared to vehicle treatment (Fig. 8B).
Figure 8.
Effect of JFE on reactive oxygen species formation in liver. A) Malondialdehyde (MDA) levels was quantified using colorimetric assay and data was presented as average of MDA levels (nmoles)/total protein concentration of tissue (mg) ± S.E.M (n=4–5 mice per group). B) Heaptic Nrf2 and Nqo1 mRNA expression. mRNA was quantified using branched DNA signal amplification assay. Data are presented as mean relative light unit (RLU) per 10μg total RNA ± S.E.M (n=4–5 mice per group). * represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05).
JFE decreases nitric oxide production and NF-kB binding
Liver inducible nitric oxide synthase (iNOS) mRNA levels were quantified as an indicator of nitric oxide production by qPCR (Fig. 9A, upper panel). BDL increased iNOS mRNA levels in vehicle-treated mice by approximately three fold. JFE treatment decreased iNOS mRNA expression in livers of mice that underwent BDL surgery. Interestingly, in shams, JFE administration markedly decreased iNOS mRNA expression to almost undetectable levels compared to vehicle-treated mice.
Figure 9.
Effect of JFE on hepatic nitric oxide production. A) Upper panel, hepatic mRNA inducible nitric oxide synthase (iNOS) mRNA expression was quantified using RT-PCR. Data are presented as mean percent control of relative mRNA expression ± S.E.M (n=4–5 mice per group). Lower panel, hepatic nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) binding activity to a consensus NF-kB response element sequence. NF-kB binding activity was quantified from pooled nuclear fractions using a TransAM assay kit and assay was performed three times. Data are presented as mean optical density (OD) at 450nm. B) Effect of JFE on LPS-induced nitrite production in RAW 264.7 cells. Inhibition of LPS-induced nitrite production by Jamun fruit extract in RAW 264.7 cells. The cells were co-treated with the indicated concentrations of JFE and 10 ng/ml LPS and incubated for 24 h. Error bars represent the standard error of three independent measurements. *represents statistically significant difference between sham and bile-duct ligated (BDL) mice of same treatment group (p≤0.05). ‡ represents statistically significant difference between JFE and vehicle treatment of same surgical group mice (p≤0.05).
NF-κB is a key transcription factor that regulates cytokine expression and promotes inflammation. Under basal conditions, NF-κB is tethered to an inhibitory protein, IκBα, in the cytoplasm (28). When activated by stimuli such as cytokines, celluar stress, and increased free radicals (12, 29), NF-κB translocates to the nucleus and upregulates transcription of several pro-inflammatory and adhesion molecules such as TNFα and Icam-1 (28). Consistent with serum and hepatic elevations in cytokine levels, BDL increased NF-κB binding in nuclear fractions isolated from both vehicle- and JFE-treated mice (Fig. 9A, lower panel). JFE treatment attenuated NF-κB binding activity by 18–20% in nuclear fractions from livers of mice that underwent sham and BDL surgery.
To investigate the inhibitory effects of JFE on inflammation, in vitro LPS-induced NO production in RAW 264.7 macrophages, was measured in culture medium. As shown in Fig. 9B, incubation of RAW 264.7 macrophages with LPS increased nitrite concentrations in the culture medium, and this nitrite production was inhibited by Jamun fruit extract in a concentration-dependent manner at JFE concentrations 20 ppm and higher.
Discussion
BDL is a common model to study cholestasis, liver injury, and fibrosis. Cholestasis causes hepatocyte necrosis by multiple mechanisms, including bile acid accumulation, generation of free radicals, oxidative stress, and inflammation (10, 30, 31). Few therapies exist for the treatment of cholestasis, which include rifampin, phenobarbital, ursodiol and bile acid binding resins (32). Multiple plant-derived phytochemicals possess therapeutic effects against diseases with an inflammatory component, such as chronic lung disease, prostate cancer, and colon cancer. Currently various antioxidants and plant-derived phytochemicals, such as resveratrol, are under investigation for their potential to elicit beneficial effects during liver disease. Treatment with antioxidants, such as quercetin and N-acetylcysteine, decreases lipid peroxidation and attenuates BDL-induced liver injury in rats (33, 34). Jamun fruit is an antioxidant rich berry fruit, which is rich in polyphenolic anthocyanin derivatives (32) and multiple studies have demonstrated that anthocyanins exhibit antioxidant activity (5, 6). The present study demonstrates a beneficial effect of Jamun on BDL-induced liver injury in mice by suppressing inflammation or lipid peroxidation. JFE was studied because anthocyanins present in Jamun are known to be more stable in a fruit extract matrix and are unstable in isolated/purified free forms. Glycosides from berry fruit extracts are stable in matrices (35).
In the present study, BDL caused hepatocellular injury as evidenced by increased serum ALT and pathological changes in liver similar to prior reports (9, 13). Livers from BDL mice typically exhibit hepatocyte necrosis, inflammation, biliary hyperplasia and fibrosis (36). Initiation of JFE treatment after BDL lowered serum ALT levels and reduced the severity of hepatocellular injury and fibrosis, indicating this extract contains phytochemicals with cytoprotective properties. Because BDL surgery causes a completely occluded bile duct, this is considered an extreme model of cholestatic liver injury and inflammation. Therefore, the fact that phytochemicals found in Jamun fruit alleviates hepatotoxicity in a BDL model is promising for further development as a nutrition-based strategy to treat liver disease (37, 38). Subsequent experiments were designed to systematically assess inflammation, lipid peroxidation, bile acid accumulation, and altered regulation of hepatobiliary transporters as the mechanism(s) by which JFE affords protection.
One of the mechanisms involved in BDL-induced liver injury is generation of reactive oxygen species and free radicals, which stimulates lipid peroxidation and disruption of cell membranes culminating in hepatocellular necrosis (6, 30, 36, 39, 40). Imbalances in oxidant/prooxidant levels in hepatocytes after BDL augments lipid peroxidation (36). MDA is a biomarker for lipid peroxidation secondary to generation of reactive oxygen species (41). In the present study, BDL increased MDA levels in livers, which was significantly decreased by JFE suggesting that suppression of lipid peroxidation is one mechanism underlying the hepatoprotective properties of JFE.
Expression of hepatobiliary transporters is coordinately altered in response to cholestatic liver injury, likely in an attempt to minimize hepatocellular levels of toxic intermediates (e.g. bile acids, lipid peroxide). An unanticipated observation was that bile acids in livers of BDL mice were higher after JFE treatment, compared to vehicle-treated BDL mice. Additional investigations were focused on the regulation of hepatobiliary transporters in an attempt to understand this observation. As described in this and other studies, uptake transporter expression is generally reduced in mice after BDL, which should lessen the bile acid burden of the liver (as reviewed by Geier et al 2007). Basolateral efflux transporters, which reduce bile acid burden in liver during cholestasis are upregulated during BDL (15). Likewise, BDL increased mRNA expression of basolateral efflux transporters such as Mrp 1, 3–6, and Ost-α and β in vehicle-treated mice. Unexpectedly, JFE treatment repressed BDL-mediated induction of Mrp1–3, 5, 6, and Ost-a and b mRNA, likely explaining the higher bile acid burden in these mice, although there is no change in Bsep protein levels. Although bile acid burden has historically been used as a marker of cholestasis, recent data demonstrate a disconnect between bile acid levels and bile flow (42). For example, hepatic bile acids are elevated in mice after partial hepatectomy, despite an increase in bile flow. As a result, we conclude that JFE-mediated protection during cholestasis is independent of bile acid homesotasis and regulation of hepatobiliary transporters. These data are in agreement with a previous study, which demonstrated that polyphenol treatment during cholestasis is hepatoprotective by a mechanism other than decreasing cholestasis (9).
Inflammation is a key event in the progression of BDL-induced liver injury, associated with increased circulating serum cytokines, activation of hepatic macrophages, and infiltration neutrophils into liver (24). Kupffer cells play a key role in inflammation during BDL-induced cholestasis by secreting cytokines and inflammatory mediators that can suppress or enhance inflammation (27, 43, 44). BDL increased macrophage and CD68 staining in livers of mice that was partially repressed by JFE. Further, profiles of serum and hepatic inflammatory mediators suggest that JFE reduced inflammation by preventing up-regulation of pro-inflammatory cytokines, such as IL-6, IL-1β, Tnf and Cxcr2, after BDL. It is important to note that JFE treatment also decreased levels of anti-inflammatory cytokines, such as IL-10 and Cxcl10. Cytokines, chemokines, and adhesion molecules that are involved in the progression of liver injury are regulated in part by the transcription factor NF-kB (45). In liver, increased NF-kB activity upregulates cytokine expression and activates hepatic stellate cells, which can promote hepatic fibrosis (46, 47). Treatment of rats with NF-kB inhibitors, such as sulfasalazine and pyrrolidine dithiocarbomate, has been shown to attenuate hepatic injury caused by BDL (47). In the present study, JFE treatment decreased NF-kB binding activity in livers of both sham and BDL mice, which corresponds with lower anti-inflammatory and pro-inflammatory cytokine levels and less macrophage infiltration. Taken together, prevention of inflammation is likely a key mechanism for the hepatoprotective effect of JFE during BDL-induced liver injury.
In addition to macrophages, neutrophils participate in the progression of liver injury during cholestasis (11) by exaggerating oxidative responses (26). Our data indicate that JFE treatment tended to reduce neutrophil recruitment in livers of BDL mice, but this finding was not statistically significant. As reviewed by Ramaiah et al., 2007, neutrophils promote liver injury in a three step process – neutrophil accumulation in liver, neutrophil extravasation into parenchyma, and adherence of neutrophils to hepatocytes, which triggers cytotoxicity (48). Each step in this process involves different cytokine signals. Tnfα, IL-1, IL-8, Cxcl1, and Cxcl2 regulate neutrophil accumulation (49, 50). Our studies demonstrate that JFE decreased Tnfα, IL-1, IL-8, Cxcl1, and Cxcl2 in mice that underwent BDL. Neutrophil extravasation into liver parenchyma from hepatic microcirculation is mediated by Icam-1 and neutrophil-cheomoattractant (CXC) chemokines (11, 51). JFE significantly decreased Icam-1, as well as CXC chemokines such as Cxcl2, in BDL mice compared to vehicle treatment. Collectively, these data suggest JFE protects against liver injury in part by reducing cytokine and chemokine pathways involved in neutrophil recruitment and extravasation. This data also supports a decrease in tissue injury with JFE treatment, although no significant decrease in liver MPO levels was observed.
Inflammation is associated with many mediators, including inducible nitric oxide synthase (iNOS), an enzyme that produces nitric oxide (NO) in activated macrophages (52, 53). The transcription factor NF-kB regulates iNOS and plays a crucial role in inflammatory and immune responses (54, 55). In this study, JFE inhibited LPS-induced nitric oxide production in a concentration-dependent manner in cultured mouse macrophages and decreased hepatic iNOS expression in sham and BDL mice. Moreover, JFE decreased BDL-induced NF-kB binding in liver. Together, these results suggest that JFE has anti-inflammatory properties that might be useful as therapeutics for cholestasis and cirrhosis.
Overall, phytochemicals present in Jamun fruit warrant additional investigation for therapeutic activity against hepatic inflammation and oxidative stress. The data presented represent the effects of a semi-purified extract, it is anticipated that additional studies using more purified chemicals will have a greater protective effect. The studies herein suggest that dietary consumption of Jamun or Jamun supplements might provide beneficial effects for liver and inflammation. In conclusion, data in the present study demonstrate that Jamun fruit contains phytochemicals that possess hepatoprotective activity against severe liver injury caused by BDL. Anthocyanin derivatives present in JFE likely elicit hepatoprotective activity through attenuating NF-kB signaling, inflammation and oxidative stress, macrophage accumulation, and lipid peroxidation.
Supplementary Material
Acknowledgments
This work was supported by NIH grants # ES-011239-01, 3R01ES016042-02S1, and also supported in part by RI-INBRE Award # P20RR016457-10 from the National Center for Research Resources (NCRR), NIH, as well as NIH grants 1R01ES016042, and 5K22ES013782. This research was supported in part by the NIEHS sponsored UMDNJ Center for Environmental Exposures and Disease, Grant #: NIEHS P30ES005022. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the NIH.
The authors would like to acknowledge Dr. Michael J. Goedken (Merck, Summit, NJ) for his help in evaluating histopathology and taking representative photomicrographs and Mr. Jeffrey Johnson (South Kingstown High School, South Kingstown, RI) for his assistance with neutrophil identification assays. The authors want to thank Verdure Sciences (Noblesville, IN) for providing freeze-dried Jamun fruit pulp powder.
Abbreviations
- ALT
alanine transaminase
- bDNA
branched DNA signal amplification
- BDL
bile duct ligation
- Bsep
bile salt-export pump
- Ccl2
Monocyte chemoattract protein-1 (Mcp-1)
- Cxcl1
keratinocyte-derived chemokines (KC)
- Cxcl2
macrophage inflammatory protein 2α (Mip-2α)
- Cxcl10
interferon gama inhibitor protein-10 (IP-10)
- Icam-1
inter-Cellular Adhesion Molecule-1
- IL
Interleukin
- JFE
jamun fruit extract
- MDA
malondialdehyde
- Mdr2
multidrug resistance protein 2
- Mrp
multidrug resistance-associated protein
- MPO
myeloperoxidase
- Nqo1
NAD(P)H quinone oxidoreductase 1
- NF-kB
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- Ntcp
sodium/taurocholate cotransporting polypeptide
- Oatp
organic anion transporting polypeptide
- PMN
polymorphonuclear leukocytes
- TBARS
Thiobarbituric acid reactive substances
- Tgfβ2
tumor growth factor beta 2
- Tnf
tumor necrosis factor
Footnotes
This work was presented in part at the annual Society of Toxicology meeting, 2009, March 15–19, Baltimore, MD.
References
- 1.Helmstadter A. Syzygium cumini (L) SKEELS (Myrtaceae) against diabetes--125 years of research. Pharmazie. 2008;63(2):91–101. [PubMed] [Google Scholar]
- 2.Benherlal PS, Arumughan C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J Sci Food Agric. 2007;87(4):2560–69. doi: 10.1002/jsfa.2957. [DOI] [PubMed] [Google Scholar]
- 3.Ravi K, Ramachandran B, Subramanian S. Effect of Eugenia Jambolana seed kernel on antioxidant defense system in streptozotocin-induced diabetes in rats. Life Sci. 2004;75(22):2717–31. doi: 10.1016/j.lfs.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Zhang Y, Seeram NP. Structure of anthocyanins from Eugenia jambolana fruit. Nat Prod Commun. 2009;4(2):217–9. [PubMed] [Google Scholar]
- 5.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8(5):362–9. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda T, Shiga K, Ohshima K, Kawakishi S, Osawa T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem Pharmacol. 1996;52(7):1033–9. doi: 10.1016/0006-2952(96)00421-2. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda T, Horio F, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside increases ex vivo oxidation resistance of serum in rats. Lipids. 1998;33(6):583–8. doi: 10.1007/s11745-998-0243-5. [DOI] [PubMed] [Google Scholar]
- 8.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339(17):1217–27. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Z, Froh M, Lehnert M, et al. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G1004–13. doi: 10.1152/ajpgi.00008.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM., Jr Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109(4):1249–56. doi: 10.1016/0016-5085(95)90585-5. [DOI] [PubMed] [Google Scholar]
- 11.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38(2):355–63. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 12.Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci. 2001;31(4):383–90. [PubMed] [Google Scholar]
- 13.Alaish SM, Torres M, Ferlito M, Sun CC, De Maio A. The severity of cholestatic injury is modulated by the genetic background. Shock. 2005;24(5):412–6. doi: 10.1097/01.shk.0000183392.83272.97. [DOI] [PubMed] [Google Scholar]
- 14.Kosters A, Karpen SJ. Bile acid transporters in health and disease. Xenobiotica. 2008;38(7–8):1043–71. doi: 10.1080/00498250802040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773(3):283–308. doi: 10.1016/j.bbamcr.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Adams LS, Chen S, Killian C, Ahmed A, Seeram NP. Eugenia jambolana Lamberry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J Agric Food Chem. 2009;57(3):826–31. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Sarma G. Study Of The Hepatoprotective Activity Of The Ethanolic Extract Of The Pulp Of Eugenia Jambolana (Jamun) In Albino Rats. J of Clin and Diagn Res. 2009;3(2):1466–74. [Google Scholar]
- 18.DeMeo M, Kolli S, Keshavarzian A, et al. Beneficial effect of a bile acid resin binder on enteral feeding induced diarrhea. Am J Gastroenterol. 1998;93(6):967–71. doi: 10.1111/j.1572-0241.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83(1):44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- 20.Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab Dispos. 2005;33(7):1062–73. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- 21.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33(9):1276–82. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol. 2007;74(11):1665–76. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denson LA, Bohan A, Held MA, Boyer JL. Organ-specific alterations in RAR alpha:RXR alpha abundance regulate rat Mrp2 (Abcc2) expression in obstructive cholestasis. Gastroenterology. 2002;123(2):599–607. doi: 10.1053/gast.2002.34758. [DOI] [PubMed] [Google Scholar]
- 24.Morita Y, Yoshidome H, Kimura F, et al. Excessive inflammation but decreased immunological response renders liver susceptible to infection in bile duct ligated mice. J Surg Res. 2008;146(2):262–70. doi: 10.1016/j.jss.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Martinez E, Perez-Alvarez V, Tsutsumi V, Shibayama M, Muriel P. Chronic bile duct obstruction induces changes in plasma and hepatic levels of cytokines and nitric oxide in the rat. Exp Toxicol Pathol. 2006;58(1):49–58. doi: 10.1016/j.etp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Jiang WG, Puntis MC, Hallett MB. Neutrophil priming by cytokines in patients with obstructive jaundice. HPB Surg. 1994;7(4):281–9. doi: 10.1155/1994/74202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy JA, Lewis H, Clements WD, et al. Kupffer cell blockade, tumour necrosis factor secretion and survival following endotoxin challenge in experimental biliary obstruction. Br J Surg. 1999;86(11):1410–4. doi: 10.1046/j.1365-2168.1999.01269.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Ye X, Steinberg H, Liu SF. Selective blockade of endothelial NF-kappaB pathway differentially affects systemic inflammation and multiple organ dysfunction and injury in septic mice. J Pathol. 220(4):490–8. doi: 10.1002/path.2666. [DOI] [PubMed] [Google Scholar]
- 29.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86(7):2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alptekin N, Mehmetcik G, Uysal M, Aykac-toker G. Evidence for oxidative stress in the hepatic mitochondria of bile duct ligated rats. Pharmacol Res. 1997;36(3):243–7. doi: 10.1006/phrs.1997.0225. [DOI] [PubMed] [Google Scholar]
- 31.Muriel P, Suarez OR, Gonzalez P, Zuniga L. Protective effect of S-adenosyl-l-methionine on liver damage induced by biliary obstruction in rats: a histological, ultrastructural and biochemical approach. J Hepatol. 1994;21(1):95–102. doi: 10.1016/s0168-8278(94)80143-6. [DOI] [PubMed] [Google Scholar]
- 32.Cies JJ, Giamalis JN. Treatment of cholestatic pruritus in children. Am J Health Syst Pharm. 2007;64(11):1157–62. doi: 10.2146/ajhp060453. [DOI] [PubMed] [Google Scholar]
- 33.Pastor A, Collado PS, Almar M, Gonzalez-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27(2):363–70. doi: 10.1016/s0168-8278(97)80183-3. [DOI] [PubMed] [Google Scholar]
- 34.Peres W, Tunon MJ, Collado PS, Herrmann S, Marroni N, Gonzalez-Gallego J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol. 2000;33(5):742–50. doi: 10.1016/s0168-8278(00)80305-0. [DOI] [PubMed] [Google Scholar]
- 35.Seeram NP, Bourquin LD, Nair MG. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J Agric Food Chem. 2001;49(10):4924–9. doi: 10.1021/jf0107508. [DOI] [PubMed] [Google Scholar]
- 36.Esrefoglu M, Gul M, Emre MH, Polat A, Selimoglu MA. Protective effect of low dose of melatonin against cholestatic oxidative stress after common bile duct ligation in rats. World J Gastroenterol. 2005;11(13):1951–6. doi: 10.3748/wjg.v11.i13.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun GY, Xia J, Xu J, et al. Dietary supplementation of grape polyphenols to rats ameliorates chronic ethanol-induced changes in hepatic morphology without altering changes in hepatic lipids. J Nutr. 1999;129(10):1814–9. doi: 10.1093/jn/129.10.1814. [DOI] [PubMed] [Google Scholar]
- 38.Gebhardt R. Oxidative stress, plant-derived antioxidants and liver fibrosis. Planta Med. 2002;68(4):289–96. doi: 10.1055/s-2002-26761. [DOI] [PubMed] [Google Scholar]
- 39.Ljubuncic P, Tanne Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut. 2000;47(5):710–6. doi: 10.1136/gut.47.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz A, Padillo FJ, Granados J, et al. Effect of melatonin on cholestatic oxidative stress under constant light exposure. Cell Biochem Funct. 2003;21(4):377–80. doi: 10.1002/cbf.1046. [DOI] [PubMed] [Google Scholar]
- 41.Baron V, Muriel P. Role of glutathione, lipid peroxidation and antioxidants on acute bile-duct obstruction in the rat. Biochim Biophys Acta. 1999;1472(1–2):173–80. doi: 10.1016/s0304-4165(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 42.Csanaky IL, Aleksunes LM, Tanaka Y, Klaassen CD. Role of hepatic transporters in prevention of bile acid toxicity after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G419–33. doi: 10.1152/ajpgi.90728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neil S, Hunt J, Filkins J, Gamelli R. Obstructive jaundice in rats results in exaggerated hepatic production of tumor necrosis factor-alpha and systemic and tissue tumor necrosis factor-alpha levels after endotoxin. Surgery. 1997;122(2):281–6. doi: 10.1016/s0039-6060(97)90019-2. discussion 86–7. [DOI] [PubMed] [Google Scholar]
- 44.Minter RM, Fan MH, Sun J, et al. Altered Kupffer cell function in biliary obstruction. Surgery. 2005;138(2):236–45. doi: 10.1016/j.surg.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang A, Schoonhoven R, Tuvia S, Brenner DA, Rippe RA. Nuclear factor kappaB in proliferation, activation, and apoptosis in rat hepatic stellate cells. J Hepatol. 2000;33(1):49–58. doi: 10.1016/s0168-8278(00)80159-2. [DOI] [PubMed] [Google Scholar]
- 47.Demirbilek S, Akin M, Gurunluoglu K, et al. The NF-kappaB inhibitors attenuate hepatic injury in bile duct ligated rats. Pediatr Surg Int. 2006;22(8):655–63. doi: 10.1007/s00383-006-1721-9. [DOI] [PubMed] [Google Scholar]
- 48.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35(6):757–66. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 49.Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1188–95. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- 50.Schlayer HJ, Laaff H, Peters T, et al. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. J Hepatol. 1988;7(2):239–49. doi: 10.1016/s0168-8278(88)80488-4. [DOI] [PubMed] [Google Scholar]
- 51.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G499–507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 52.Chung JW, Noh EJ, Zhao HL, et al. Anti-inflammatory activity of prosapogenin methyl ester of platycodin D via nuclear factor-kappaB pathway inhibition. Biol Pharm Bull. 2008;31(11):2114–20. doi: 10.1248/bpb.31.2114. [DOI] [PubMed] [Google Scholar]
- 53.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6(12):3051–64. [PubMed] [Google Scholar]
- 54.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45(1):7–17. [PubMed] [Google Scholar]
- 55.O’Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5(7):549–63. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.