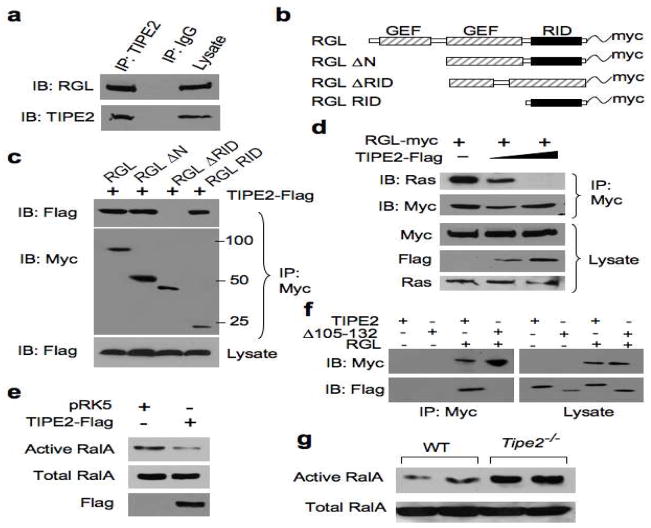
Figure 1. TIPE2 binds the Ras-interacting domain of RGL, and inhibits Ral activation.
(a) Interaction between endogenous RGL and TIPE2. Raw 264.7 cell lysates were immunoprecipitated (IP) with mouse anti-TIPE2 or control IgG, and subjected to SDS-PAGE and immunoblotting (IB). (b) Schematics of the RGL constructs used in this study. GEF, guanine nucleotide exchange factor; RID, Ras-interacting domain. (c) Interaction of TIPE2 with RGL Ras-interacting region. Lysates of 293T cells transiently transfected with RGL constructs and a TIPE2-Flag construct were immunoprecipitated with anti-Myc, and subjected to SDS-PAGE and IB. (d) TIPE2 prevents Ras from binding to RGL. Lysates of 293T cells transiently transfected with RGL-Myc (2 μg/10-cm plate) and increasing amounts of TIPE2-Flag constructs (5 μg to 10 μg/10-cm plate) were immunoprecipitated with anti-Myc, and subjected to SDS-PAGE and IB. (e) 293T cells were transiently transfected with TIPE2-Flag or pRK5 plasmids. 18 hours later, cell lysates were subjected to pull-down using GST-RalBP1 RBD. RalA from the pull-down and total RalA in the lysates were determined by IB. (f) TIPE2 Δ105–132 does not bind RGL. 293T cells were transielntly transfected with either TIPE2-Flag or TIPE2 Δ105–132 constructs, with or without RGL plasmid. 18 hours later, cell lysates were immunoprecipitated with anti-Myc antibody, and subjected to SDS-PAGE and IB. (g) Active and total RalA levels in wild type and Tipe2−/− bone marrow-derived murine macrophages were determined as in panel (e). Data shown are representative of 3 independent experiments.