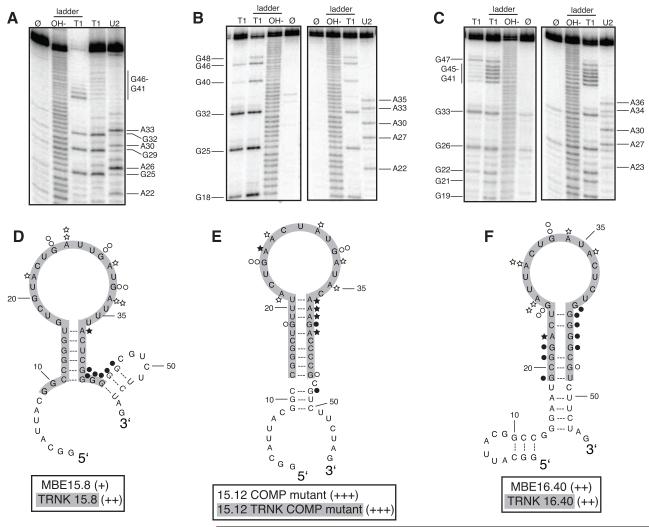
Figure 4. Secondary structure model and binding affinity of full length and truncated RNA aptamers to RVFV N.
A. to C. Structure probing of full length RNA aptamers MBE15.8 (A), 15.12 COMP mutant (B) and MBE16.40 (C) was conducted to determine the reactivity of guanosine (lane T1) and adenosine (lane U2) residues to enzymes RNase T1 and RNase U2. Lane Ø represents undigested RNA. T1 and OH− ladders are used as RNA sequencing lanes. D. to F. Summary of nucleotide reactivity superimposed on secondary structure models of MBE 15.8 (D) 15.12COMP mutant (E) MBE16.40 (F). Circles represent results obtained from experiments conducted with the single-strand guanosine specific T1 nuclease and stars are representative of the single-strand adenosine specific U2 nuclease. The greater the number of symbols associated with a specific nucleotide, the stronger the reactivity. Open symbols indicate a lack of reactivity. Truncated aptamers are highlighted in gray. Aptamers were rated based on percent of input RNA bound at 12.5 μM N: 40-49% = (+), 50-59% = (++), and 60-69% = (+++).