Abstract
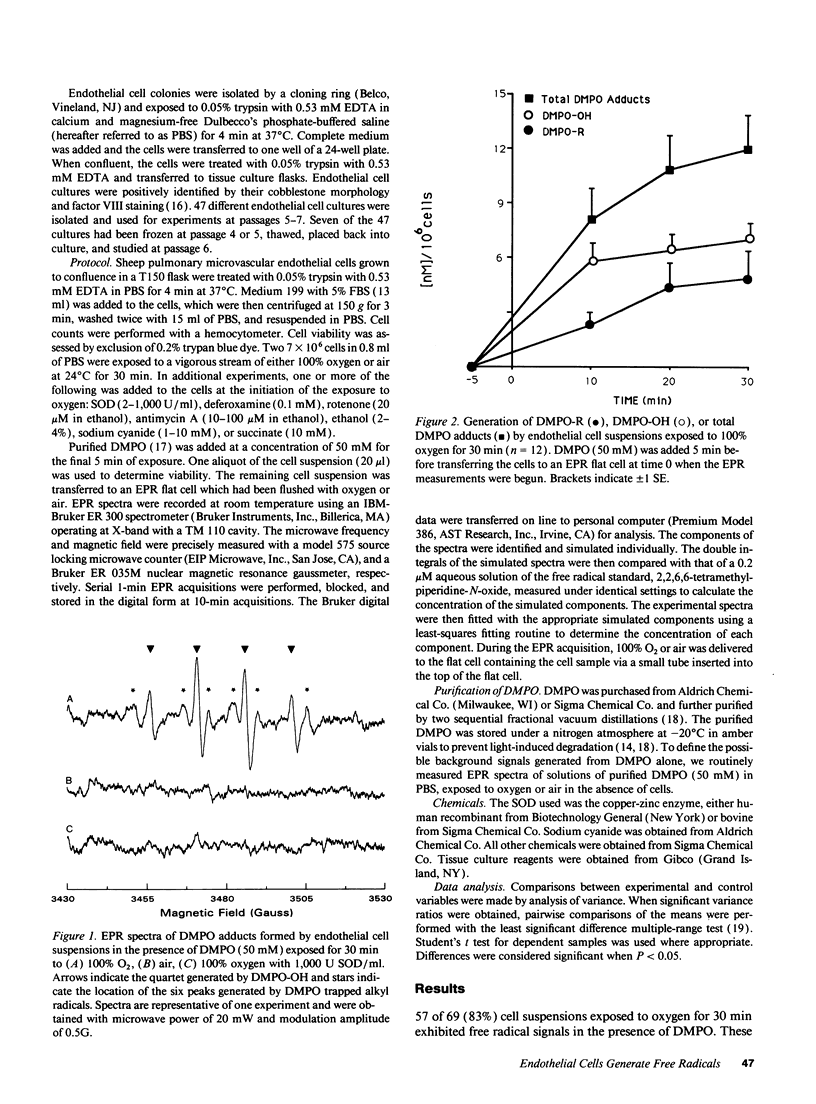
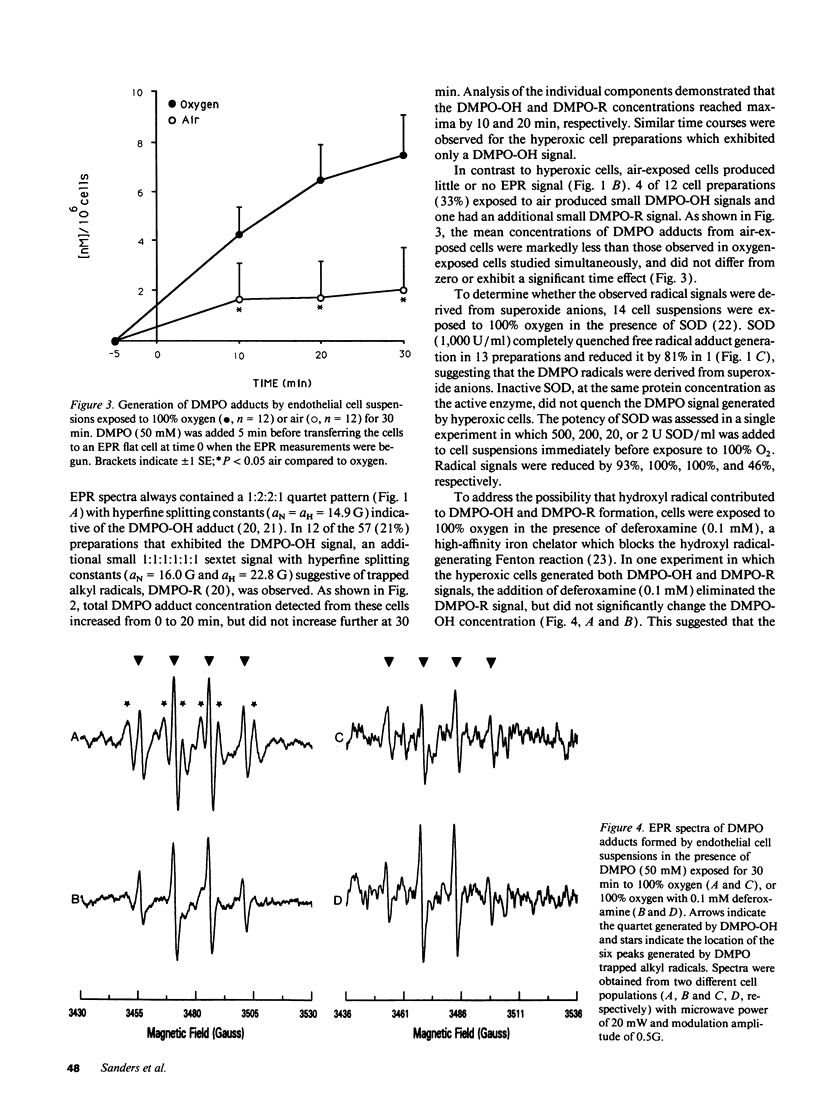
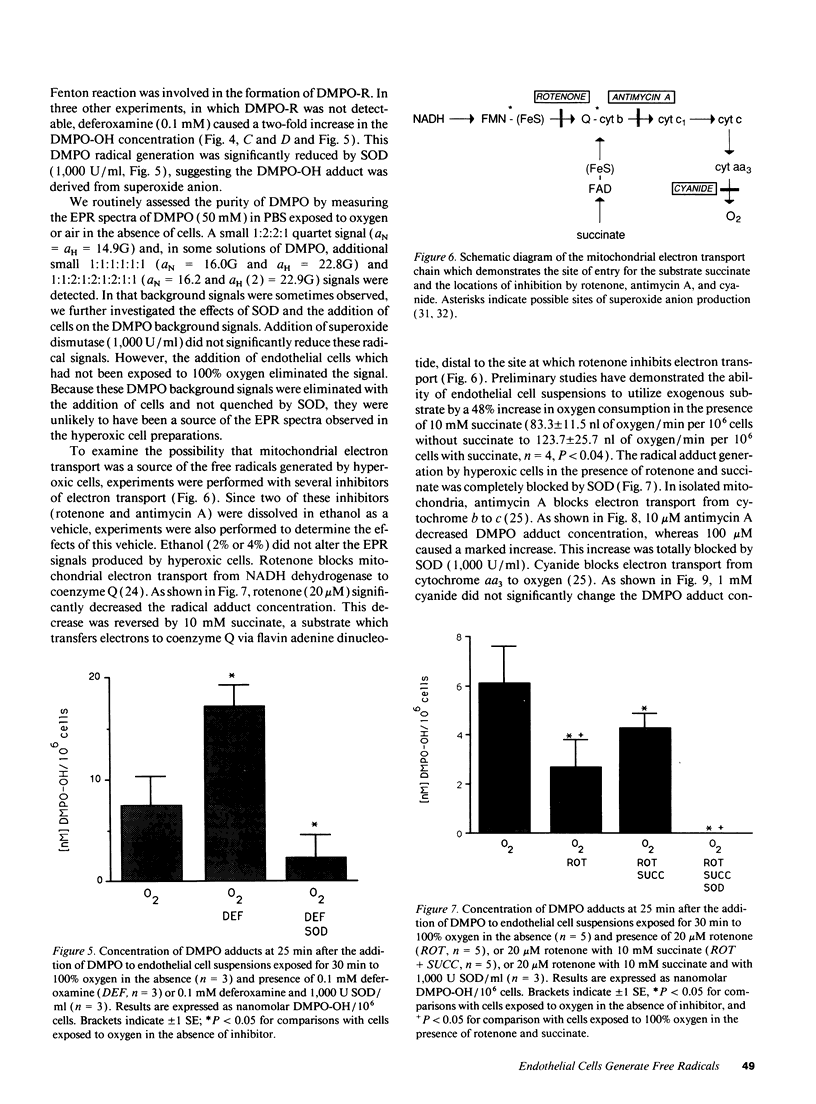
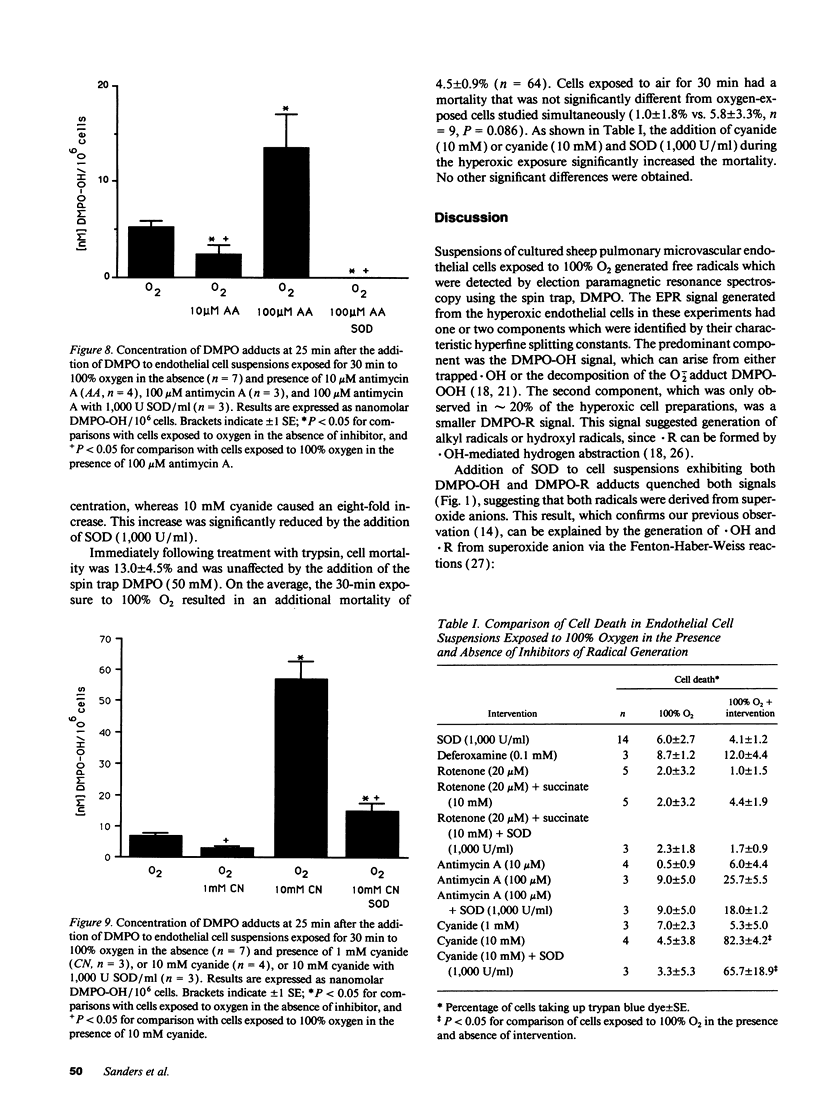
Free radical generation by hyperoxic endothelial cells was studied using electron paramagnetic resonance (EPR) spectroscopy and the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO). Studies were performed to determine the radical species produced, whether mitochondrial electron transport was involved, and the effect of the radical generation on cell mortality. Sheep pulmonary microvascular endothelial cell suspensions exposed to 100% O2 for 30 min exhibited prominent DMPO-OH and, occasionally, additional smaller DMPO-R signals thought to arise from the trapping of superoxide anion (O2-.), hydroxyl (.OH), and alkyl (.R) radicals. Superoxide dismutase (SOD) quenched both signals suggesting that the observed radicals were derived from O2-.. Studies with deferoxamine suggested that the generation of .R occurred secondary to the formation of .OH from O2-. via an iron-mediated Fenton reaction. Blocking mitochondrial electron transport with rotenone (20 microM) markedly decreased radical generation. Cell mortality increased slightly in oxygen-exposed cells. This increase was not significantly altered by SOD or deferoxamine, nor was it different from the mortality observed in air-exposed cells. These results suggest that endothelial cells exposed to hyperoxia for 30 min produce free radicals via mitochondrial electron transport, but under the conditions of these experiments, this radical generation did not appear cause cell death.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop C. T., Mirza Z., Crapo J. D., Freeman B. A. Free radical damage to cultured porcine aortic endothelial cells and lung fibroblasts: modulation by culture conditions. In Vitro Cell Dev Biol. 1985 Apr;21(4):229–236. doi: 10.1007/BF02620934. [DOI] [PubMed] [Google Scholar]
- Block E. R., Patel J. M., Angelides K. J., Sheridan N. P., Garg L. C. Hyperoxia reduces plasma membrane fluidity: a mechanism for endothelial cell dysfunction. J Appl Physiol (1985) 1986 Mar;60(3):826–835. doi: 10.1152/jappl.1986.60.3.826. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Butler E. N., Repine J. E. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis. 1983 Sep;128(3):469–472. doi: 10.1164/arrd.1983.128.3.469. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Chai Y., Cohen M. S. Do human neutrophils make hydroxyl radical? Determination of free radicals generated by human neutrophils activated with a soluble or particulate stimulus using electron paramagnetic resonance spectrometry. J Biol Chem. 1986 Apr 5;261(10):4426–4431. [PubMed] [Google Scholar]
- Buettner G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Jornot L., Junod A. F. Response of human endothelial cell antioxidant enzymes to hyperoxia. Am J Respir Cell Mol Biol. 1992 Jan;6(1):107–115. doi: 10.1165/ajrcmb/6.1.107. [DOI] [PubMed] [Google Scholar]
- Jornot L., Mirault M. E., Junod A. F. Protein synthesis in hyperoxic endothelial cells: evidence for translational defect. J Appl Physiol (1985) 1987 Aug;63(2):457–464. doi: 10.1152/jappl.1987.63.2.457. [DOI] [PubMed] [Google Scholar]
- Junod A. F., Petersen H., Jornot L. Thymidine kinase, thymidylate synthase, and endothelial cell growth under hyperoxia. J Appl Physiol (1985) 1987 Jan;62(1):10–14. doi: 10.1152/jappl.1987.62.1.10. [DOI] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Ellis E. F., Jenkins L. W., Povlishock J. T., Rowe G. T., Hess M. L. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ Res. 1985 Jul;57(1):142–151. doi: 10.1161/01.res.57.1.142. [DOI] [PubMed] [Google Scholar]
- Markey B. A., Phan S. H., Varani J., Ryan U. S., Ward P. A. Inhibition of cytotoxicity by intracellular superoxide dismutase supplementation. Free Radic Biol Med. 1990;9(4):307–314. doi: 10.1016/0891-5849(90)90005-4. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Hoover R., Jones M. R., Berry L. C., Jr, Brigham K. L. In vitro effects of endotoxin on bovine and sheep lung microvascular and pulmonary artery endothelial cells. J Cell Physiol. 1989 Jan;138(1):165–174. doi: 10.1002/jcp.1041380122. [DOI] [PubMed] [Google Scholar]
- Michiels C., Toussaint O., Remacle J. Comparative study of oxygen toxicity in human fibroblasts and endothelial cells. J Cell Physiol. 1990 Aug;144(2):295–302. doi: 10.1002/jcp.1041440216. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. Superoxide-dependent redox cycling of citrate-Fe3+: evidence for a superoxide dismutaselike activity. Arch Biochem Biophys. 1987 Feb 15;253(1):257–267. doi: 10.1016/0003-9861(87)90659-x. [DOI] [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Palluy O., Bonne C., Modat G. Hypoxia/reoxygenation alters endothelial prostacyclin synthesis--protection by superoxide dismutase. Free Radic Biol Med. 1991;11(3):269–275. doi: 10.1016/0891-5849(91)90123-k. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. B., Housset B., Jean-Mairet Y., Junod A. F. Effects of hyperoxia on biochemical indexes of pig aortic endothelial function. In Vitro. 1983 Aug;19(8):625–634. doi: 10.1007/BF02619576. [DOI] [PubMed] [Google Scholar]
- Samuni A., Samuni A., Swartz H. M. The cellular-induced decay of DMPO spin adducts of .OH and .O2. Free Radic Biol Med. 1989;6(2):179–183. doi: 10.1016/0891-5849(89)90115-9. [DOI] [PubMed] [Google Scholar]
- Smith L. J. Hyperoxic lung injury: biochemical, cellular, and morphologic characterization in the mouse. J Lab Clin Med. 1985 Sep;106(3):269–278. [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Duke S. S., Kuppusamy P., Sylvester J. T., Gabrielson E. W. Electron paramagnetic resonance evidence that cellular oxygen toxicity is caused by the generation of superoxide and hydroxyl free radicals. FEBS Lett. 1989 Jul 31;252(1-2):12–16. doi: 10.1016/0014-5793(89)80881-6. [DOI] [PubMed] [Google Scholar]


