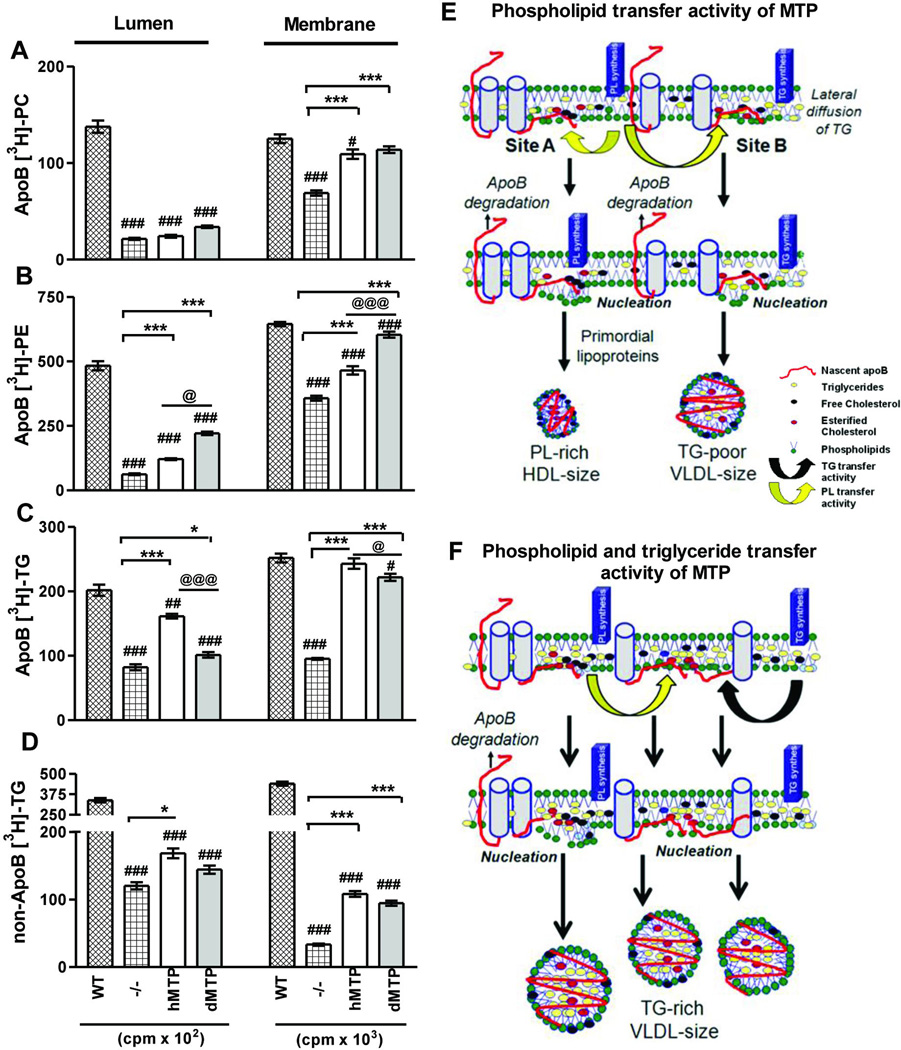
Figure 8. Subcellular partitioning of newly synthesized lipids in hMTP and dMTP expressing mice.
(A–D) Primary hepatocytes were incubated with [3H]-oleate (10 µCi/ml) for 30 minutes. ApoB-associated lumenal and membrane phosphatidylcholine (PC) (A); phosphatidylethanolamine (PE) (B); triglycerides (C) and triglycerides not associated with apoB (D) are shown. Mean ± SEM, n=3. ***p < 0. 001, **p < 0. 01, *p < 0. 05. (E–F) Model of lipoprotein biosynthesis: Nascent apoB (red) is shown to traverse the ER membrane and interact with inner leaflet to form “nucleation sites” or retro-translocated for degradation. Phospholipid transfer activity in dMTP can bring phospholipid molecules to nascent apoB (yellow arrows) and help in the formation of nucleation sites. Nucleation site A has fewer triglycerides. Desorption from this site may yield phospholipid-rich apoB48-containing HDL size particles. At site B, few triglyceride molecules are shown to arrive via lateral diffusion. Desorption from this site might result in the synthesis of triglyceride-poor apoB100 and apoB48-containing VLDL size lipoproteins (E). Triglyceride transfer activity (black arrow) can bring neutral lipids creating triglyceride enriched nucleation sites, lipidate nascent apoB, and prevent proteasomal degradation. Desorption from these sites might yield heterogeneous apoB100 and apoB48 containing VLDL-size particles (F).