Figure 6.
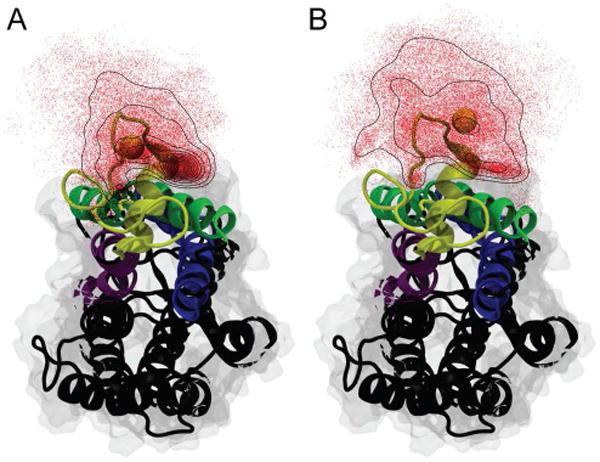
View of rhodopsin looking along the membrane normal from the cytoplasmic side (corresponding to the cell interior) toward the extracellular side. The relative positions of the terminal methyl groups of the palmitoyl substituents attached to Cys322 (A) and Cys323 (B) were determined in 1-ns intervals. Their distribution is shown as red dots with black contour lines indicating their density. For reference purposes in both panels the average position of the cysteine residue to which the lipid modification is attached is shown as a yellow vdW sphere. Helices H1 (green), H2 (blue), H7 (purple), and H8 (yellow) are color-coded with the remaining helices shown in black. The palmitoylation at Cys322 shows a clear accumulation at the protein/lipid interface. In contrast the palmitoylation at Cys323 is more evenly distributed about the cysteine residue and only shows minor accumulation close to helix H1. Rhodopsin was rendered using VMD67.
