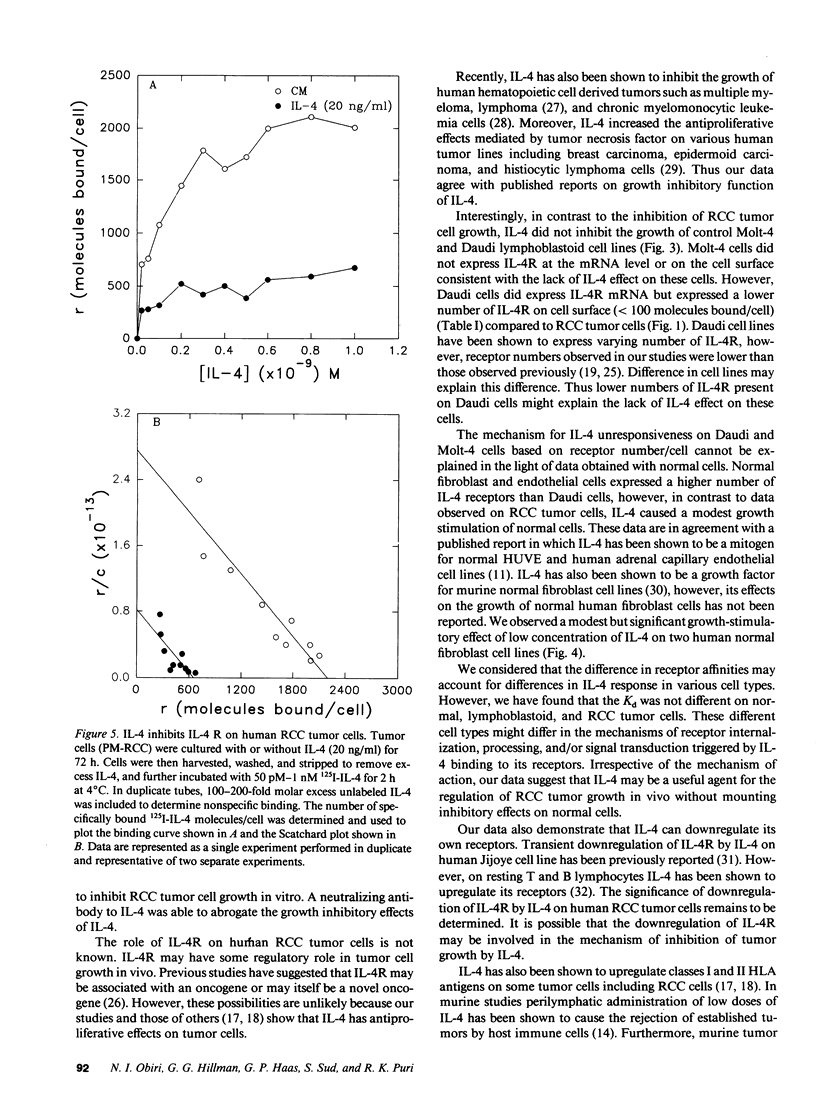
Abstract
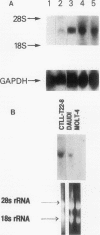
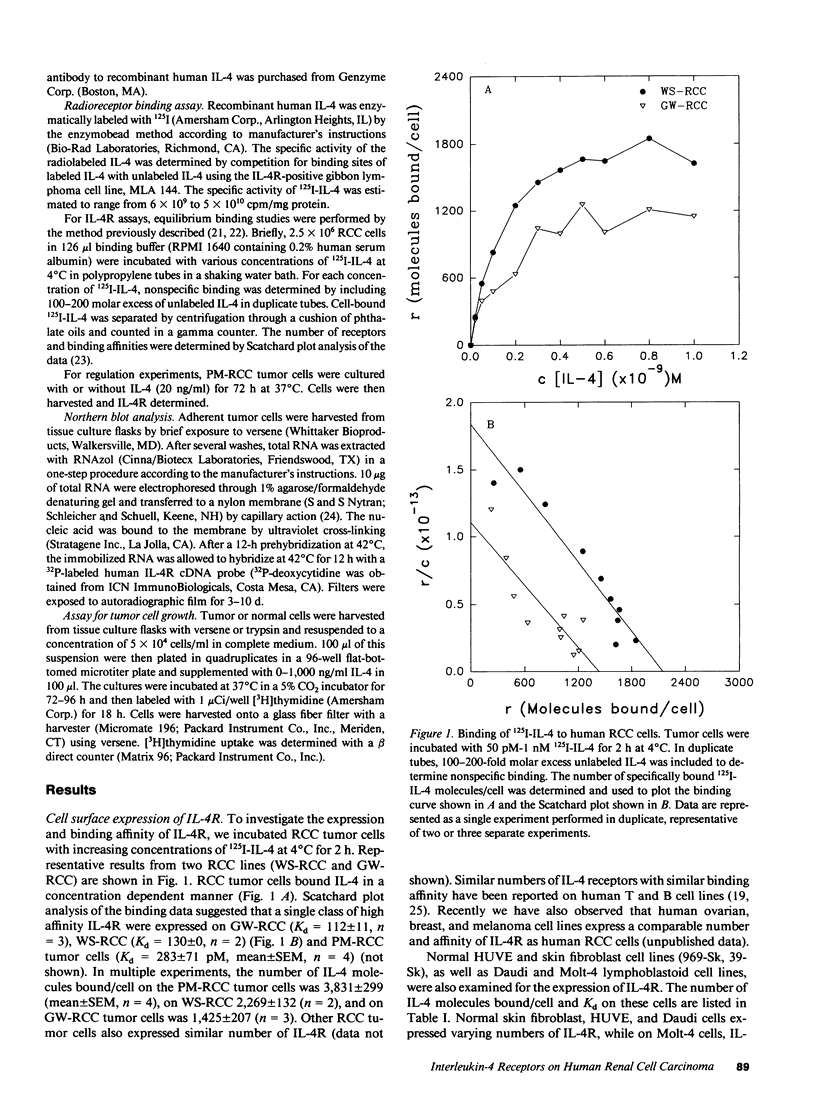
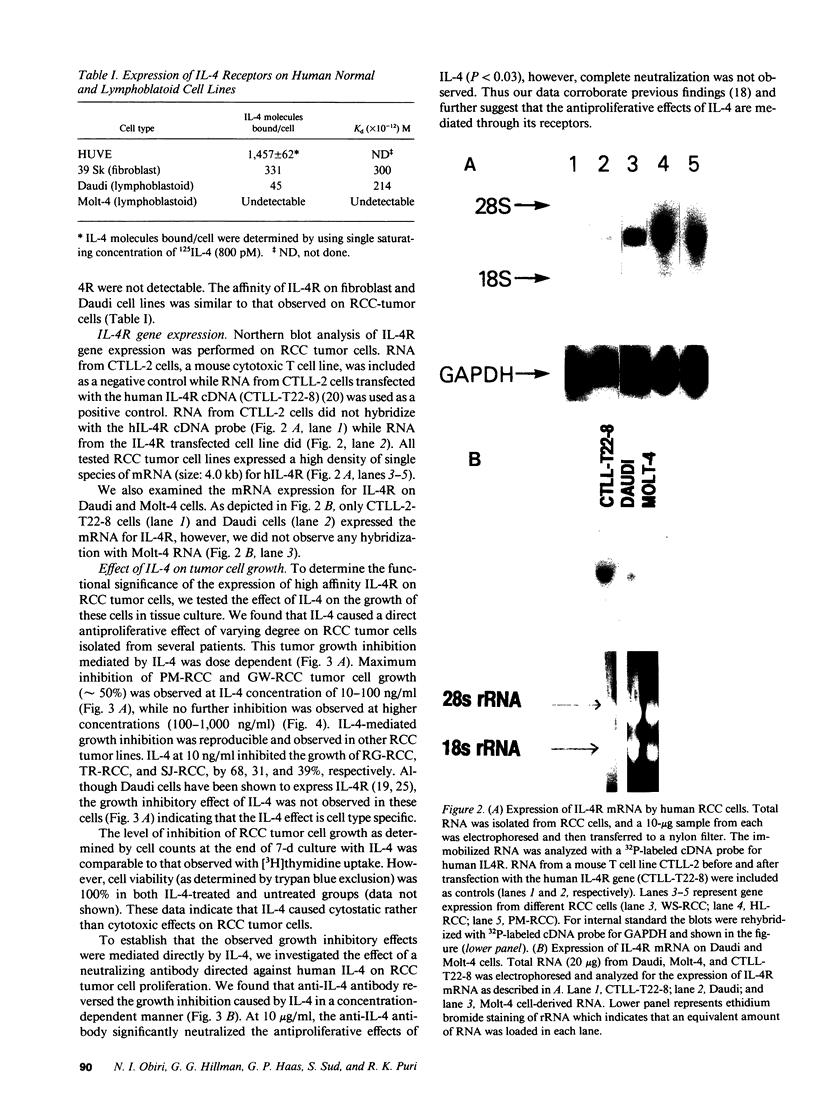
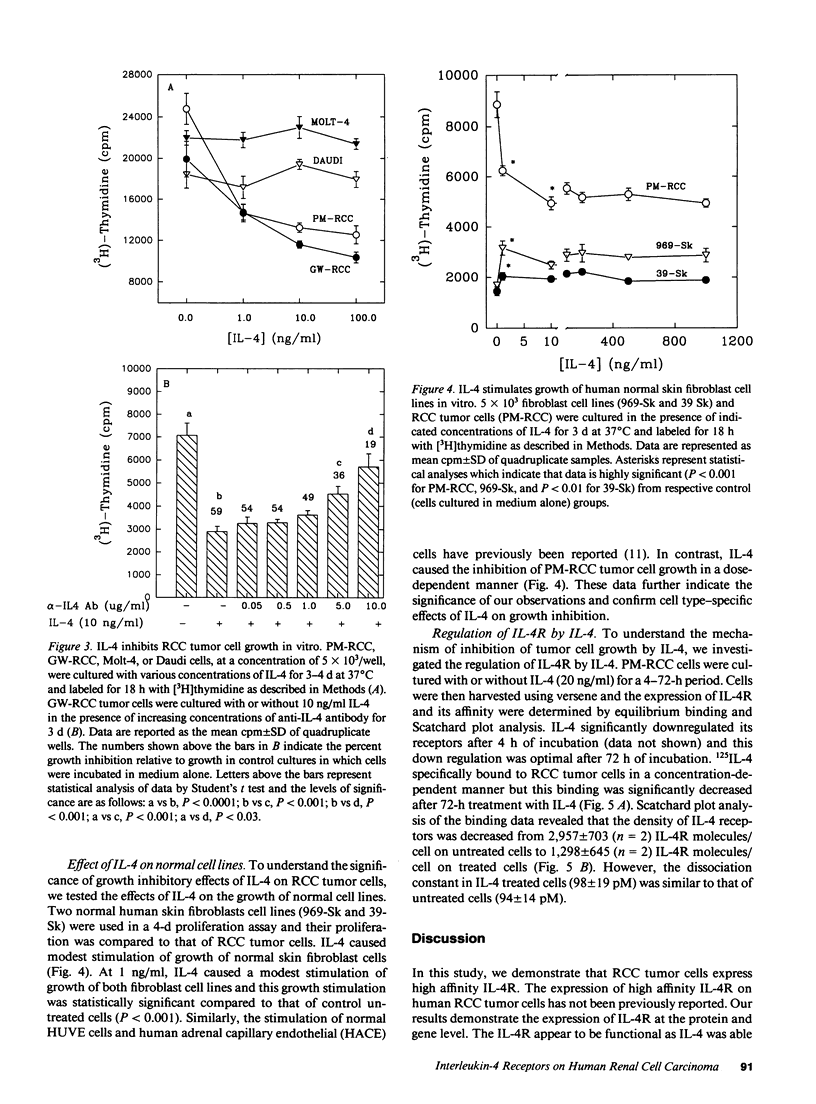
Previously, Puri et al. (Puri, R. K., M. Ogata, P. Leland, G. M. Feldman, D. Fitzgerald, and I. Pastan. 1991. Cancer Res. 51:3011-3017) have demonstrated that murine sarcoma and colon adenocarcinoma cells express high affinity interleukin-4 receptors (IL-4R) which are internalized after binding to a chimeric ligand consisting of IL-4 and Pseudomonas exotoxin. In the present study, we have tested primary cultures of human renal cell carcinoma (RCC) cells, generated from tumor specimens obtained after nephrectomy, for the expression of IL-4R and their modulation by IL-4. By using iodinated IL-4 in a receptor binding assay, we observed that renal cell carcinoma cells expressed a single class of high affinity IL-4R ranging from 1,425 +/- 207 (mean +/- SEM) to 3,831 +/- 299 (mean +/- SEM) IL-4R molecules/cell with a Kd ranging from 112 +/- 11 pM to 283 +/- 71 pM. Northern blot analysis for IL-4R gene expression, performed with a cDNA probe to IL-4R, revealed that all RCC cells exhibited a single mRNA species of 4 kb. IL-4 downregulated the surface expression of IL-4R on one RCC tumor cell line. The function of IL-4R expression on RCC tumor cells was further determined by investigating the effect of IL-4 on tumor cell growth in vitro and comparing it with IL-4 effect on growth of normal fibroblast and endothelial cell lines. Tumor cell growth, as measured by [3H]thymidine incorporation, was inhibited by IL-4 from 20 to 68% in a dose-dependent manner. A neutralizing antibody to human IL-4 was able to reverse the growth inhibitory effect of IL-4. Normal human fibroblast and endothelial cell lines also expressed high affinity IL-4R, however, IL-4 did not inhibit their growth in vitro. In fact, IL-4 caused modest stimulation of their growth. Taken together, our findings can help develop strategies for the treatment of RCC in which IL-4R may be used as a target for IL-4 itself, for IL-4 toxin therapy or, alternatively, in gene therapy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi K., Shibuya T., Harada M., Takamatsu Y., Uike N., Eto T., Niho Y. Interleukin 4 suppresses the spontaneous growth of chronic myelomonocytic leukemia cells. J Clin Invest. 1991 Jul;88(1):223–230. doi: 10.1172/JCI115281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Jabaari B., Ladyman H. M., Larché M., Sivolapenko G. B., Epenetos A. A., Ritter M. A. Elevated expression of the interleukin 4 receptor in carcinoma: a target for immunotherapy? Br J Cancer. 1989 Jun;59(6):910–914. doi: 10.1038/bjc.1989.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco M., Giovarelli M., Forni M., Modesti A., Scarpa S., Masuelli L., Forni G. Low doses of IL-4 injected perilymphatically in tumor-bearing mice inhibit the growth of poorly and apparently nonimmunogenic tumors and induce a tumor-specific immune memory. J Immunol. 1990 Nov 1;145(9):3136–3143. [PubMed] [Google Scholar]
- Brown M., Hu-Li J., Paul W. E. IL-4/B cell stimulatory factor 1 stimulates T cell growth by an IL-2-independent mechanism. J Immunol. 1988 Jul 15;141(2):504–511. [PubMed] [Google Scholar]
- Cabrillat H., Galizzi J. P., Djossou O., Arai N., Yokota T., Arai K., Banchereau J. High affinity binding of human interleukin 4 to cell lines. Biochem Biophys Res Commun. 1987 Dec 31;149(3):995–1001. doi: 10.1016/0006-291x(87)90507-9. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Galizzi J. P., Zuber C. E., Cabrillat H., Djossou O., Banchereau J. Internalization of human interleukin 4 and transient down-regulation of its receptor in the CD23-inducible Jijoye cells. J Biol Chem. 1989 Apr 25;264(12):6984–6989. [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon D. S., Banez M., Okun E., Morton D. L., Irie R. F. Modulation of human melanoma cells by interleukin-4 and in combination with gamma-interferon or alpha-tumor necrosis factor. Cancer Res. 1991 Apr 15;51(8):2002–2008. [PubMed] [Google Scholar]
- Hoon D. S., Okun E., Banez M., Irie R. F., Morton D. L. Interleukin 4 alone and with gamma-interferon or alpha-tumor necrosis factor inhibits cell growth and modulates cell surface antigens on human renal cell carcinomas. Cancer Res. 1991 Oct 15;51(20):5687–5693. [PubMed] [Google Scholar]
- Horohov D. W., Crim J. A., Smith P. L., Siegel J. P. IL-4 (B cell-stimulatory factor 1) regulates multiple aspects of influenza virus-specific cell-mediated immunity. J Immunol. 1988 Dec 15;141(12):4217–4223. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Swisher S. G., Minehart E. H., McBride W. H., Economou J. S. Interleukin-4 downregulates interleukin-6 production in human peripheral blood mononuclear cells. J Leukoc Biol. 1990 May;47(5):475–479. doi: 10.1002/jlb.47.5.475. [DOI] [PubMed] [Google Scholar]
- Milanese C., Richardson N. E., Reinherz E. L. Identification of a T helper cell-derived lymphokine that activates resting T lymphocytes. Science. 1986 Mar 7;231(4742):1118–1122. doi: 10.1126/science.2935936. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Haldar S., Prystowsky M. B., Lammie P. Lymphokine regulation of inflammatory processes: interleukin-4 stimulates fibroblast proliferation. Clin Immunol Immunopathol. 1988 Nov;49(2):292–298. doi: 10.1016/0090-1229(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Sassenfeld H. M., Urdal D. L. Characterization of the human B cell stimulatory factor 1 receptor. J Exp Med. 1987 Aug 1;166(2):476–488. doi: 10.1084/jem.166.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Puri R. K., Finbloom D. S., Leland P., Mostowski H., Siegel J. P. Expression of high-affinity IL-4 receptors on murine tumour infiltrating lymphocytes and their up-regulation by IL-2. Immunology. 1990 Aug;70(4):492–497. [PMC free article] [PubMed] [Google Scholar]
- Puri R. K., Ogata M., Leland P., Feldman G. M., FitzGerald D., Pastan I. Expression of high-affinity interleukin 4 receptors on murine sarcoma cells and receptor-mediated cytotoxicity of tumor cells to chimeric protein between interleukin 4 and Pseudomonas exotoxin. Cancer Res. 1991 Jun 1;51(11):3011–3017. [PubMed] [Google Scholar]
- Taylor C. W., Grogan T. M., Salmon S. E. Effects of interleukin-4 on the in vitro growth of human lymphoid and plasma cell neoplasms. Blood. 1990 Mar 1;75(5):1114–1118. [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Toi M., Harris A. L., Bicknell R. Interleukin-4 is a potent mitogen for capillary endothelium. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1287–1293. doi: 10.1016/0006-291x(91)91561-p. [DOI] [PubMed] [Google Scholar]
- Totpal K., Aggarwal B. B. Interleukin 4 potentiates the antiproliferative effects of tumor necrosis factor on various tumor cell lines. Cancer Res. 1991 Aug 15;51(16):4266–4270. [PubMed] [Google Scholar]