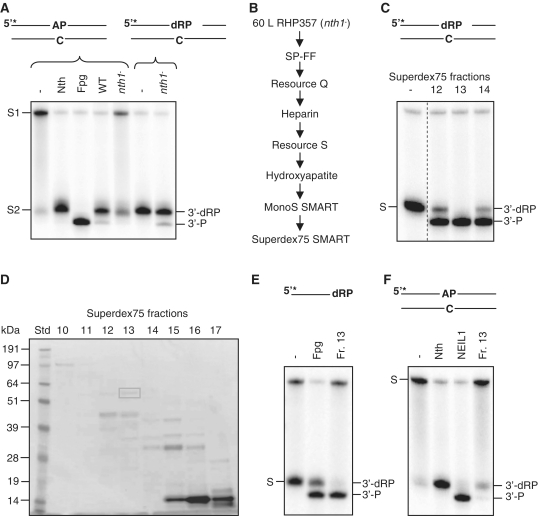
Figure 1.
Identification and characterization of endogenous Tdp1. (A) Cleavage of intact and nicked AP substrates in S. pombe whole cell extracts. Two micrograms of total protein extracts from FY526 (wt) and RHP357 (nth1−) cells were incubated with 10 fmol 5′-[32P]-labeled duplex DNA containing an AP site (opposite C) in reaction buffer for 30 min at 37°C (left panel). Two micrograms of the RHP357 (nth1−) extract was used in an equivalent reaction using the same substrate pretreated with Nth (right panel). The cleavage products were separated by 20% denaturing PAGE and visualized by phosphorimaging. The DNA substrates with an intact (S1) or Nth-nicked (S2) AP site and the cleavage products (3′-dRP and 3′-P) are indicated. Escherichia coli Nth and Fpg were used as positive controls for migration of the 3′-dRP and 3′-P products, respectively. (B) Schematic illustration of the columns used in the purification/identification process. (C) δ-elimination activity in Superdex75 SMART fractions. One microliter of fraction 12–14 from Superdex75 was incubated with Nth-nicked ds AP substrate and analyzed for cleavage activity as described in Figure 1A. The substrate (S; 3′-dRP) and the cleavage product (3′-P) are indicated. All samples were run on the same gel, but the control lane was cut from another part of the gel as indicated by the dotted line. (D) SDS–PAGE of Superdex75 SMART fractions. Proteins in fractions 10–17 (22 µl) from Superdex75 were separated by 10% SDS–PAGE, and bands in fraction 13 were excised from the gel and analyzed by MS. (E) δ-cleavage activity of Nth-nicked ss AP substrate. One microliter of fraction 13 from Superdex75 was incubated with an Nth-nicked ss AP substrate and analyzed for cleavage as described in Figure 1A. The substrate (S; 3′-dRP) and the cleavage product (3′-P) are indicated. Escherichia coli Fpg was used as a positive control for 3′-P terminus. (F) Assay for AP lyase activity. One microliter of fraction 13 from Superdex75 was incubated with an intact ds AP substrate and analyzed for cleavage as described in Figure 1A. The substrate (S) and the cleavage products (3′-dRP and 3′-P) are indicated. Escherichia coli Nth and purified human NEIL1 were used as positive controls for 3′-dRP and 3′-P, respectively.