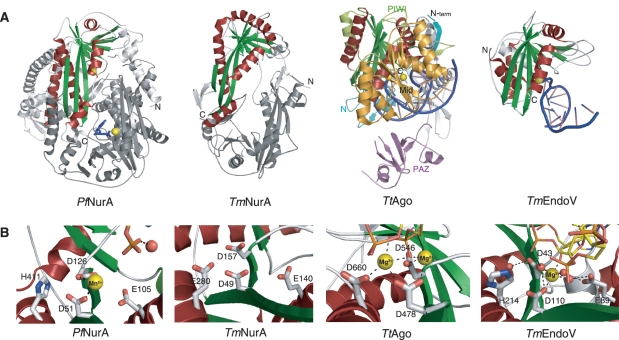
Figure 5.
Structural comparison of RNaseH-like fold proteins. (A) Comparison of the Pf NurA, TmNurA (1zup), TtAgo (3hvr) and TmEndo (2w35) structures. Conserved α-helices (red) and β-sheets (green) form a PIWI domain. Pf NurA and TmNurA show a dimeric structure, whose interfaces significantly differ from each other. The TmNurA structure does not contain any metal ions in its active site. The monomer structures of TtAgo and TmEndoV are shown and their metal ions are denoted as yellow spheres. DNA is shown in blue. (B) Comparison of active site in Pf NurA, TmNurA, TtAgo and TmEndoV. His411 of Pf NurA is only conserved in TmEndoV and replaced to Asp in other structures. Oxygen and nitrogen atoms are shown in red and blue, respectively. A water molecule is in red sphere.