Abstract
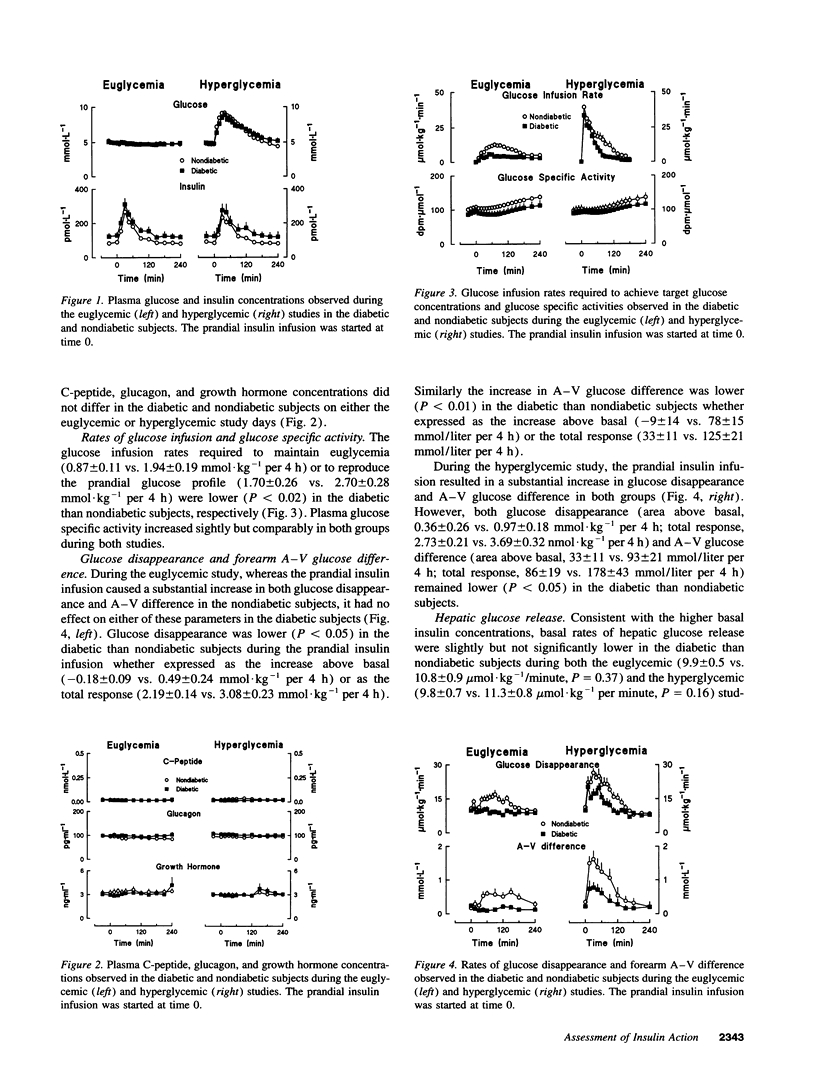
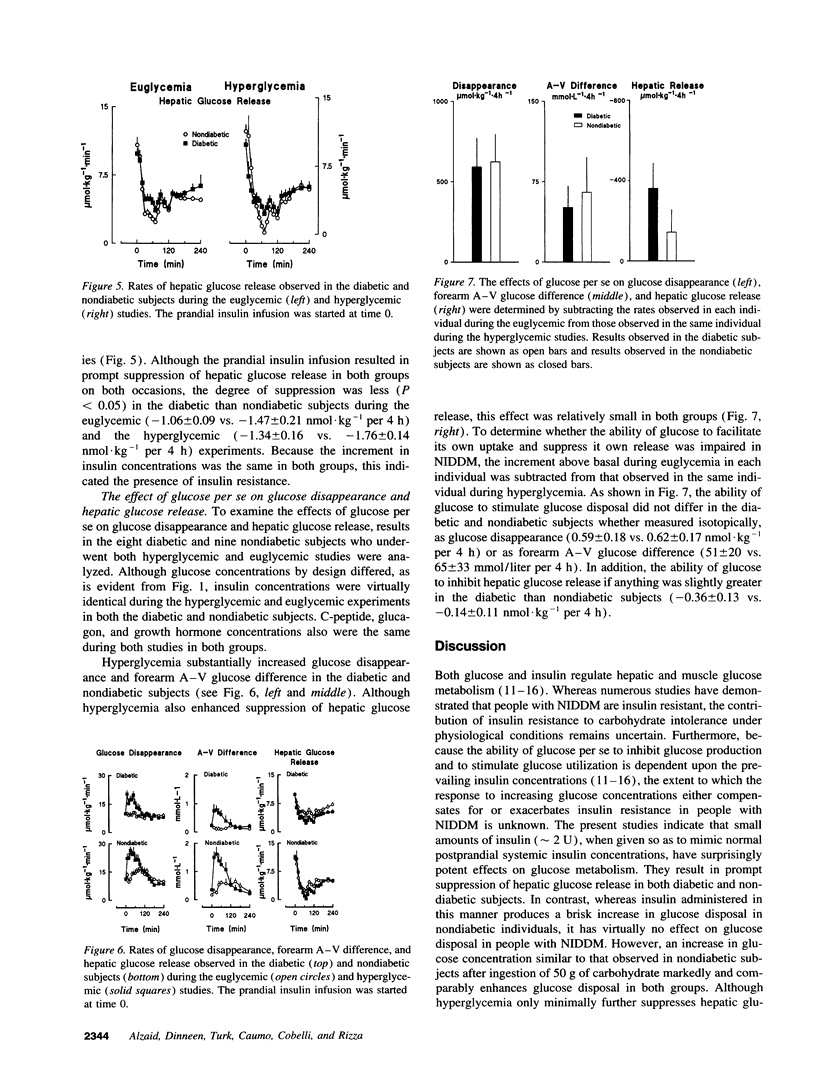
Insulin concentrations in humans continuously change and typically increase only when glucose also increases such as with eating. In this setting, it is not known whether the severity of hepatic and extrahepatic insulin resistance is comparable and whether the ability of glucose to regulate its own uptake and release is defective in non-insulin-dependent diabetes mellitus (NIDDM). To address this question, NIDDM and nondiabetic subjects were studied when glucose concentrations were clamped at either 5 mM (euglycemia) or varied so as to mimic the glucose concentrations observed in nondiabetic humans after food ingestion (hyperglycemia). Insulin was infused so as to simulate a "nondiabetic" postprandial profile. During euglycemia, insulin increased glucose disposal in nondiabetic but not diabetic subjects indicating marked extrahepatic resistance. In contrast, insulin-induced suppression of glucose release was only minimally less (P < 0.05) in diabetic than nondiabetic subjects (-1.06 +/- 0.09 vs. -1.47 +/- 0.21 nmol.kg-1 per 4 h). Hyperglycemia substantially enhanced disposal in both groups. Glucose effectiveness measured as the magnitude of enhancement of disposal (0.59 +/- 0.18 vs. 0.62 +/- 0.17 nmollkg-1 per 4 h) and suppression of release (-0.36 +/- 0.12 vs. -0.14 +/- 0.12 nmol.kg-1 per 4 h) did not differ in the diabetic and nondiabetic subjects. In conclusion, when assessed in the presence of a physiological insulin profile, people with NIDDM demonstrate: (a) profound extrahepatic insulin resistance, (b) modest hepatic insulin resistance, and (c) normal ability of glucose to stimulate its own uptake and suppress its own release.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron A. D., Kolterman O. G., Bell J., Mandarino L. J., Olefsky J. M. Rates of noninsulin-mediated glucose uptake are elevated in type II diabetic subjects. J Clin Invest. 1985 Nov;76(5):1782–1788. doi: 10.1172/JCI112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman R. N., Finegood D. T., Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985 Winter;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- Best J. D., Taborsky G. J., Jr, Halter J. B., Porte D., Jr Glucose disposal is not proportional to plasma glucose level in man. Diabetes. 1981 Oct;30(10):847–850. doi: 10.2337/diab.30.10.847. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. C., Poulin R. A., Bergman R. N. Dynamics of hepatic and peripheral insulin effects suggest common rate-limiting step in vivo. Diabetes. 1993 Feb;42(2):296–306. doi: 10.2337/diab.42.2.296. [DOI] [PubMed] [Google Scholar]
- Bradley D. C., Steil G. M., Bergman R. N. Quantitation of measurement error with Optimal Segments: basis for adaptive time course smoothing. Am J Physiol. 1993 Jun;264(6 Pt 1):E902–E911. doi: 10.1152/ajpendo.1993.264.6.E902. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Block N. E., Buse M. G. Structural differences between liver- and muscle-derived insulin receptors in rats. J Biol Chem. 1986 Nov 5;261(31):14361–14364. [PubMed] [Google Scholar]
- Butler P. C., Caumo A., Zerman A., O'Brien P. C., Cobelli C., Rizza R. A. Methods for assessment of the rate of onset and offset of insulin action during nonsteady state in humans. Am J Physiol. 1993 Apr;264(4 Pt 1):E548–E560. doi: 10.1152/ajpendo.1993.264.4.E548. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Caumo A., Zerman A., O'Brien P. C., Cobelli C., Rizza R. A. Methods for assessment of the rate of onset and offset of insulin action during nonsteady state in humans. Am J Physiol. 1993 Apr;264(4 Pt 1):E548–E560. doi: 10.1152/ajpendo.1993.264.4.E548. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Kryshak E. J., Marsh M., Rizza R. A. Effect of insulin on oxidation of intracellularly and extracellularly derived glucose in patients with NIDDM. Evidence for primary defect in glucose transport and/or phosphorylation but not oxidation. Diabetes. 1990 Nov;39(11):1373–1380. doi: 10.2337/diab.39.11.1373. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Rizza R. A. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes. 1991 Jan;40(1):73–81. [PubMed] [Google Scholar]
- Butler P., Kryshak E., Rizza R. Mechanism of growth hormone-induced postprandial carbohydrate intolerance in humans. Am J Physiol. 1991 Apr;260(4 Pt 1):E513–E520. doi: 10.1152/ajpendo.1991.260.4.E513. [DOI] [PubMed] [Google Scholar]
- Campbell P. J., Mandarino L. J., Gerich J. E. Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism. 1988 Jan;37(1):15–21. doi: 10.1016/0026-0495(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Capaldo B., Santoro D., Riccardi G., Perrotti N., Saccà L. Direct evidence for a stimulatory effect of hyperglycemia per se on peripheral glucose disposal in type II diabetes. J Clin Invest. 1986 Apr;77(4):1285–1290. doi: 10.1172/JCI112432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Raju S. M., Sinha M. K., Goldfine I. D., Dohm G. L. Heterogeneity of human liver, muscle, and adipose tissue insulin receptor. Biochem Biophys Res Commun. 1988 Feb 29;151(1):123–129. doi: 10.1016/0006-291x(88)90567-0. [DOI] [PubMed] [Google Scholar]
- Chen C., Williams P. F., Caterson I. D. Liver and peripheral tissue glycogen metabolism in obese mice: effect of a mixed meal. Am J Physiol. 1993 Nov;265(5 Pt 1):E743–E751. doi: 10.1152/ajpendo.1993.265.5.E743. [DOI] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Dimitrakoudis D., Vranic M., Klip A. Effects of hyperglycemia on glucose transporters of the muscle: use of the renal glucose reabsorption inhibitor phlorizin to control glycemia. J Am Soc Nephrol. 1992 Nov;3(5):1078–1091. doi: 10.1681/ASN.V351078. [DOI] [PubMed] [Google Scholar]
- Doeden B., Rizza R. Use of a variable insulin infusion to assess insulin action in obesity: defects in both the kinetics and amplitude of response. J Clin Endocrinol Metab. 1987 May;64(5):902–908. doi: 10.1210/jcem-64-5-902. [DOI] [PubMed] [Google Scholar]
- ELIAS H. A re-examination of the structure of the mammalian liver; the hepatic lobule and its relation to the vascular and biliary systems. Am J Anat. 1949 Nov;85(3):379-456, 15 pl. doi: 10.1002/aja.1000850303. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Simonson D. C., Katz L. D., Reichard G., Jr, Bevilacqua S., Barrett E. J., Olsson M., DeFronzo R. A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988 Jan;37(1):79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Rizza R. A. Effects of tolazamide and exogenous insulin on insulin action in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1986 May 15;314(20):1280–1286. doi: 10.1056/NEJM198605153142003. [DOI] [PubMed] [Google Scholar]
- Giacca A., Fisher S. J., Shi Z. Q., Gupta R., Lickley H. L., Vranic M. Importance of peripheral insulin levels for insulin-induced suppression of glucose production in depancreatized dogs. J Clin Invest. 1992 Nov;90(5):1769–1777. doi: 10.1172/JCI116051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen I. L., Cryer P. E., Rizza R. A. Comparison of insulin-mediated and glucose-mediated glucose disposal in patients with insulin-dependent diabetes mellitus and in nondiabetic subjects. Diabetes. 1985 Aug;34(8):751–755. doi: 10.2337/diab.34.8.751. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Behmand R. A., Arafah B. M. Chronic hyperglycemia increases the density of glucose transporters in human erythrocyte membranes. J Clin Endocrinol Metab. 1991 Apr;72(4):814–818. doi: 10.1210/jcem-72-4-814. [DOI] [PubMed] [Google Scholar]
- Kahn B. B. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992 May;89(5):1367–1374. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz H., Homan M., Jensen M., Caumo A., Cobelli C., Rizza R. Assessment of insulin action in NIDDM in the presence of dynamic changes in insulin and glucose concentration. Diabetes. 1994 Feb;43(2):289–296. doi: 10.2337/diab.43.2.289. [DOI] [PubMed] [Google Scholar]
- Kellerer M., Sesti G., Seffer E., Obermaier-Kusser B., Pongratz D. E., Mosthaf L., Häring H. U. Altered pattern of insulin receptor isotypes in skeletal muscle membranes of type 2 (non-insulin-dependent) diabetic subjects. Diabetologia. 1993 Jul;36(7):628–632. doi: 10.1007/BF00404072. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mokan M., Mandarino L. J. Intracellular defects in glucose metabolism in obese patients with NIDDM. Diabetes. 1992 Jun;41(6):698–706. doi: 10.2337/diab.41.6.698. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie L., Dimitrakoudis D., Marette A., Annabi B., Klip A., Vranic M., van de Werve G. Opposite effects of hyperglycemia and insulin deficiency on liver glycogen synthase phosphatase activity in the diabetic rat. Diabetes. 1993 Feb;42(2):363–366. doi: 10.2337/diab.42.2.363. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Consoli A., Kelley D. E., Reilly J. J., Nurjhan N. Fasting hyperglycemia normalizes oxidative and nonoxidative pathways of insulin-stimulated glucose metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1990 Dec;71(6):1544–1551. doi: 10.1210/jcem-71-6-1544. [DOI] [PubMed] [Google Scholar]
- Matthews D. R., Naylor B. A., Jones R. G., Ward G. M., Turner R. C. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes. 1983 Jul;32(7):617–621. doi: 10.2337/diab.32.7.617. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Pallardo F. V., Seidner G. A., Vannucci S., Simpson I. A., Birnbaum M. J. Kinetics of GLUT1 and GLUT4 glucose transporters expressed in Xenopus oocytes. J Biol Chem. 1993 Apr 25;268(12):8514–8520. [PubMed] [Google Scholar]
- Paolisso G., Scheen A. J., Giugliano D., Sgambato S., Albert A., Varricchio M., D'Onofrio F., Lefèbvre P. J. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man: importance of pulse frequency. J Clin Endocrinol Metab. 1991 Mar;72(3):607–615. doi: 10.1210/jcem-72-3-607. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Fink R., Griffin J., Olefsky J. M., Kolterman O. G. Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J Clin Invest. 1984 Mar;73(3):664–672. doi: 10.1172/JCI111258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A., Barzilai N., Howard K., Sebel G., Hu M. Mechanism by which hyperglycemia inhibits hepatic glucose production in conscious rats. Implications for the pathophysiology of fasting hyperglycemia in diabetes. J Clin Invest. 1993 Sep;92(3):1126–1134. doi: 10.1172/JCI116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Shulman R. G., Shulman G. I. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Apr;89(4):1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà L., Cryer P. E., Sherwin R. S. Blood glucose regulates the effects of insulin and counterregulatory hormones on glucose production in vivo. Diabetes. 1979 Jun;28(6):533–536. doi: 10.2337/diab.28.6.533. [DOI] [PubMed] [Google Scholar]
- Shuster L. T., Go V. L., Rizza R. A., O'Brien P. C., Service F. J. Incretin effect due to increased secretion and decreased clearance of insulin in normal humans. Diabetes. 1988 Feb;37(2):200–203. doi: 10.2337/diab.37.2.200. [DOI] [PubMed] [Google Scholar]
- Vaag A., Henriksen J. E., Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Mar;89(3):782–788. doi: 10.1172/JCI115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch S., Gebhart S. S., Bergman R. N., Phillips L. S. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990 Dec;71(6):1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- White N. H., Skor D., Santiago J. V. Practical closed-loop insulin delivery. A system for the maintenance of overnight euglycemia and the calculation of basal insulin requirements in insulin-dependent diabetics. Ann Intern Med. 1982 Aug;97(2):210–213. doi: 10.7326/0003-4819-97-2-210. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Raskin P., Aydin I., Unger R. Effects of insulin on the response of immunoreactive glucagon to an intravenous glucose load in human diabetes. Metabolism. 1979 May;28(5):568–574. doi: 10.1016/0026-0495(79)90199-9. [DOI] [PubMed] [Google Scholar]
- Yang Y. J., Hope I. D., Ader M., Bergman R. N. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989 Nov;84(5):1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. H., Youn M. S., Bergman R. N. Synergism of glucose and fructose in net glycogen synthesis in perfused rat livers. J Biol Chem. 1986 Dec 5;261(34):15960–15969. [PubMed] [Google Scholar]


