Figure 4.
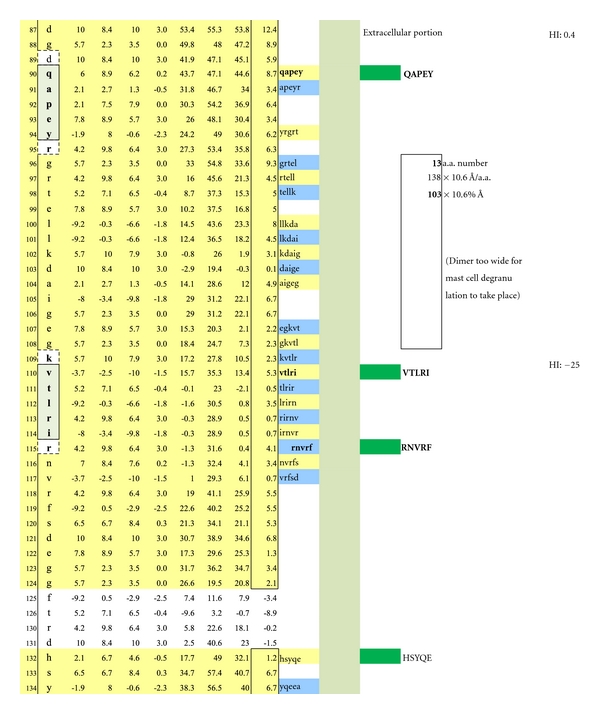
Displayed is the measured distance between two IgE autoantibodies if each was to bind a potential epitopic dimer site (QAPEY and VTLRI) with each site incorporating five, uniquely sequenced, contiguous amino acids flanked on either end by a nonreactive, normally present amino acid thus making a 7 amino acid, antibody binding footprint. Each intervening amino acid between epitopes is estimated to be 10.6 Ångströms in width. When the interfootprint dimer distance analysis is performed, the potential dimer between QAPEY and VTLRI is inadequate for mast cell degranulation because there are 13 intervening amino acids between the two epitopes, and this is equivalent to a distance of 103 Ångströms, which is 3 Ångströms above the mandated upper limit of 100 Ångströms. HI: peptide hydrophilic index.
