Table 2.
Preparation of isothiocyanatesa.
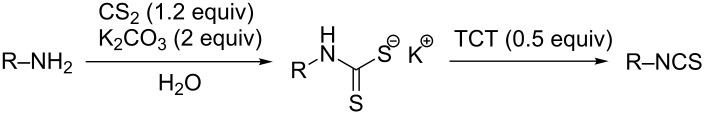 | ||||
| entry | substrate | product | timeb (h) | yieldc (%) |
| 1 | 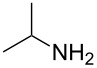 |
 |
1 | 85 |
| 2 |  |
 |
1 | 94 |
| 3 | 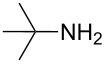 |
 |
1 | 80 |
| 4 |  |
 |
1 | 95 |
| 5 |  |
 |
1 | 98 |
| 6 | 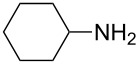 |
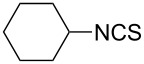 |
1 | 95 |
| 7 |  |
 |
1 | 99 |
| 8 | 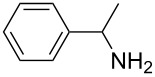 |
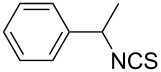 |
1 | 99 |
| 9 | 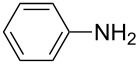 |
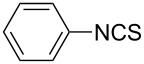 |
3 | 98 (94) |
| 10 |  |
 |
3 | 86 |
| 11 | 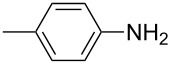 |
 |
3 | 93 |
| 12 | 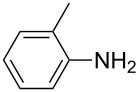 |
 |
3 | 95 |
| 13 | 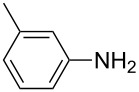 |
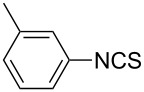 |
3 | 98 |
| 14 | 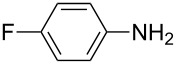 |
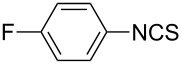 |
12 | 81d |
| 15 |  |
 |
20 | 70d |
aReaction conditions: 20 mmol of amine substrate, 24 mmol of CS2, 40 mmol of K2CO3, room temperature. HPLC monitored the conversion. After the amine was totally consumed, the mixture was cooled to 0 °C, and 10 mmol of TCT in CH2Cl2 was added dropwise. The mixture was stirred for another 0.5 h and basified to pH >11 with 6 N NaOH. bThe reaction time for the first step. cIsolated yield. Data in the parenthesis is the isolated yield in 1-mol scale. d3.0 equiv of CS2 was used and the reaction temperature was 40 °C.
