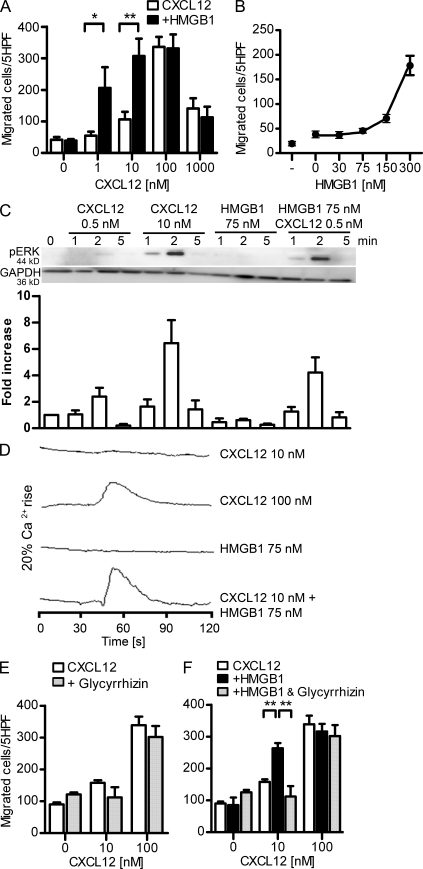
Figure 2.
HMGB1 increases CXCL12-induced activities in human monocytes and CXCR4-transfected cells and is sensitive to glycyrrhizin. (A) Chemotaxis toward increasing concentrations of CXCL12 ± 300 nM HMGB1. Migrated cells were counted per five high-power fields (HPF) and are shown as mean ± SEM of three independent experiments performed with cells from different donors (*, P < 0.05; **, P < 0.01, ANOVA plus Bonferroni posttest). (B) Dose–response curve of HMGB1 in the presence of 10 nM CXCL12. Migrated cells were counted per five high-power fields and are shown as mean ± SEM of three independent experiments. (C) Time course of ERK1&2 phosphorylation induced by CXCL12 alone or in the presence of HMGB1. The intensity of pERK1&2 bands was analyzed by densitometry, normalized with matching GAPDH, and compared with the unstimulated control. One representative blot is shown; the bars and error bars represent mean ± SEM of four independent experiments with cells from different donors. (D) Changes in [Ca2+]i were monitored in cells loaded with 50 nM Fura-2-AM and stimulated with CXCL12 and HMGB1. One representative set of measurements out of three independent experiments is shown. (E and F) Chemotaxis toward increasing concentrations of CXCL12 ± 200 µM glycyrrhizin ± 300 nM HMGB1. In all these experiments, migrated monocytes were counted per five high-power fields. Bars and error bars represent mean ± SEM of three different experiments performed with monocytes from different donors (**, P < 0.01, ANOVA plus Bonferroni posttest).