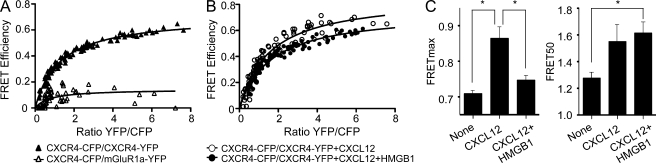
Figure 5.
CXCL12 and the CXCL12–HMGB1 heterocomplex trigger different conformational changes in CXCR4 homodimers. (A and B) FRET saturation curves were generated in HEK293T cells transiently cotransfected with a constant amount of CXCR4-CFP (CXCR4-C; 2.0 µg; ∼300,000 FU) and increasing quantities of CXCR4-YFP (CXCR4-Y; 0.25–4.25 µg; ∼80,000-2,000,000 FU) or mGluR1α-Y (0.5–6.0 µg; ∼110,000-2,100,000 FU) as negative control. The curves represent data obtained in eight independent experiments. (B) Effect of CXCL12 (100 nM, 30 min) or CXCL12 + HMGB1 (100 nM and 300 nM, 30 min) on CXCR4 homodimers. The curves represent data obtained in 12 independent experiments. (C) FRETmax and FRET50 values shown were deduced using a nonlinear regression equation applied to a single binding site model and are representative of 8–12 independent experiments. FRETmax signals for CXCL12-treated and CXCL12–HMGB1-treated cells increase significantly compared with untreated cells (*, P < 0.05). Furthermore, the FRETmax value for CXCL12-treated cells is significantly higher (*, P < 0.05) than the FRETmax for HMGB1–CXCL12-treated cells. Error bars indicate SEM.