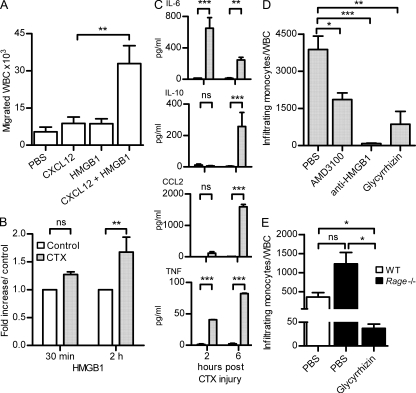
Figure 6.
Migration of WBCs in vivo depends on the HMGB1–CXCL12 heterocomplex. (A) WBCs were collected from air pouches 6 h after injection of PBS, 10 pmol CXCL12, 300 pmol HMGB1, or CXCL12 + HMGB1. Bars and error bars represent mean ± SEM of cell influx from at least six mice per condition (**, P < 0.01, ANOVA plus Dunnett’s test). (B) HMGB1–CXCL12 heterocomplex detected by hybrid ELISA in the muscles injured with CTX and in the untreated contralateral ones. Results are expressed as fold increase of the complex in CTX-treated muscle compared with the untreated controls and normalized to the total weight of muscle. Mean ± SEM of three independent experiments is shown (**, P < 0.01, ANOVA plus Bonferroni posttest). (C) Mouse cytokines were measured at 2 and 6 h after injury in muscles treated with CTX and in the untreated contralateral ones (control). Results are expressed as picogram/milliliter of cytokines detected with cytometric bead assay and normalized to the total weight of tissue. Mean ± SEM of three independent experiments is shown (**, P < 0.01; ***, P < 0.001, ANOVA plus Bonferroni posttest). (D) Mononuclear CD11bhigh+ cells infiltrating the muscle of WT BALB/c mice were counted 6 h after CTX injury and normalized against circulating total leukocytes (WBC). Mice were treated with PBS, AMD3100, α-HMGB1 antibody, or glycyrrhizin as described in Materials and methods. Mean ± SEM of infiltrating cells from at least three mice per condition is shown (*, P < 0.05; **, P < 0.01; ***, P < 0.005, ANOVA plus Dunnett’s posttest). (E) Mononuclear CD11bhigh+ cells infiltrating the muscles of Rage−/− mice were counted 6 h after CTX injury and normalized against circulating total leukocytes (WBC). Mice were treated with PBS or glycyrrhizin before injury. Mean ± SEM of infiltrating cells from three mice is shown (*, P < 0.05, ANOVA plus Dunnett’s posttest).