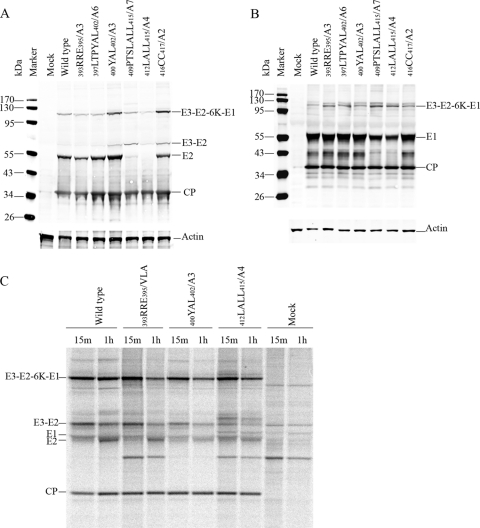
Fig 1.
Analysis of translation and processing of the structural polyprotein. (A) Processing of E2 glycoprotein in BHK-15 cells electroporated with in vitro-transcribed RNA from the wild type (SINV) or mutant clones. At 12 h posttransfection, the cells were harvested and equal amounts of proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. The Western blots were processed with SINV-specific rabbit polyclonal anti-E2 and anti-CP and mouse monoclonal anti-actin primary antibodies and infrared-labeled (IRDye 680 and IRDye 800) goat anti-rabbit and goat anti-mouse secondary antibodies. (B) Processing of E1 glycoprotein in BHK-15 cells electroporated with in vitro-transcribed RNA from wild-type (SINV) or mutant clones done as for panel A, except that the proteins were separated by 12% SDS-PAGE gel and the Western blots were processed with SINV-specific rabbit polyclonal anti-E1 and anti-CP and mouse monoclonal anti-actin primary antibodies. (C) Pulse-chase analysis of structural polyprotein processing. BHK-15 cells were electroporated with in vitro-transcribed RNA from the wild-type and selected nonbudding mutant clones. At 12 h postelectroporation, the cells were pulsed with [35S]methionine for 15 min and chased for 1 h (pulse, 15 min; chase, 1 h). The cells were lysed and cell lysates were immunoprecipitated using SINV-specific rabbit E2 polyclonal antibody and subjected to 10% Bis-Tris gel SDS-PAGE and autoradiography.