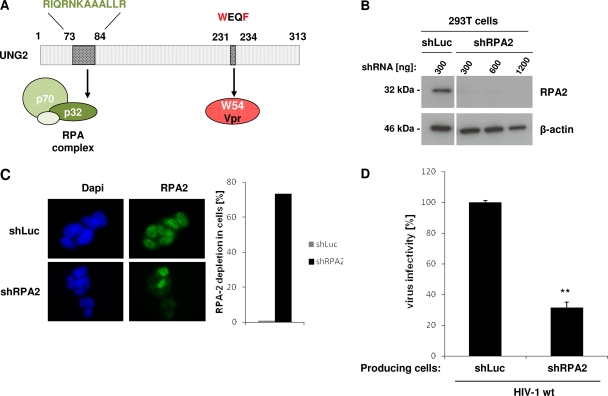
Fig 6.
Impact of RPA2 depletion on virus infectivity. (A) Schematic representation of UNG2 showing the motifs required for interaction with Vpr or with the p32 subunit (RPA2) of the RPA heterotrimeric complex. (B and C) Depletion of RPA2 in virus-producing cells. 293T cells were transduced with lentiviruses expressing shRNA against either RPA2 or Luciferase used as a control. In panel B, lysates from shRNA-transduced cells were analyzed by Western blotting with anti-RPA2 (top) and anti-β-actin (bottom) antibodies. In panel C, shRNA-transduced cells were analyzed by indirect immunofluorescence with anti-RPA2 antibody (left part, right panels), and the number of shRNA-transduced cells without nuclear RPA2 staining was quantified over 100 cells (right part). Nuclei were stained with DAPI (left part, left panels). Cells were analyzed by epifluorescence microscopy, and images were acquired with a charge-coupled device camera. (D) Virus infectivity. Wild-type GFP reporter viruses were produced in shRNA-transduced 293T cells as indicated and then used to infect HeLa-CD4 cells. Viruses were normalized for CAp24 before infection. The percentages of GFP-positive infected cells were measured by flow cytometry 60 h later. Infectivity was normalized to that of viruses produced in shLuc-transduced cells and measured on HeLa-CD4 as target cells. Values are the means of three independent experiments. Error bars represent one SD from the mean. Statistical significance was determined by using the Student t test (n.s., P > 0.05; *, P < 0.05; **, P < 0.01).