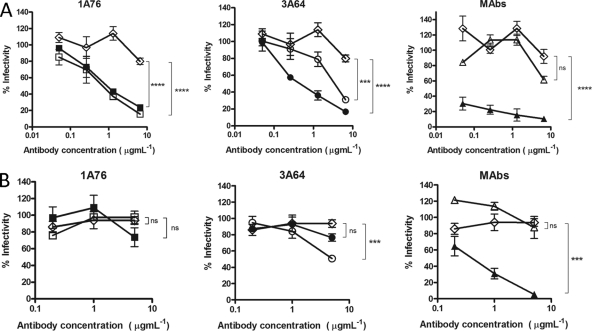
Fig 5.
Human and murine antibodies that target regions of E2 encompassing aa 412 to 423 and aa 434 to 446 neutralize HCVpp entry but differ in their neutralization breadths. Dilutions of 1A76 peptide I-Ig (■), 3A64 peptide I-Ig (●), 1A76 peptide II-Ig (□), 3A64 peptide II-Ig (○), MAb AP33 (▲), or MAb 2/69a (△) were used to neutralize the infectivity of H77c HCVpp (A) or JFH-1 HCVpp (B). Two different negative controls were used for the neutralization assays (♢): a normal human serum sample mock purified using the magnetic bead process was used as a negative control in the Ig neutralization assays, whereas HIV-1-specific monoclonal antibody 2F5 was used as a negative control in the MAb neutralization assays. Although neutralization assays were performed at the same time, for clarity, they are plotted on two panels corresponding to each peptide Ig and a third panel for the monoclonal antibodies. The same negative-control NHS data set is included in both Ig panels. The mean infectivities observed at the highest concentrations of each test antibody and the negative-control antibody were compared by using a t test. P values are indicated as follows: ns, P > 0.05; *, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The mean luminescences observed for the uninhibited H77c HCVpp and JFH-1 HCVpp were 3,830 relative light units (RLU) and 3,819 RLU, and those for the no-env controls were 165 RLU and 138 RLU, respectively.