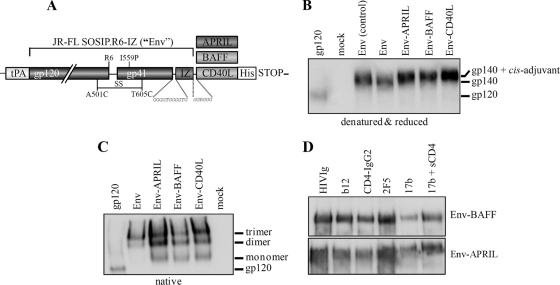
Fig 1.
Chimeric Env-APRIL, Env-BAFF, and Env-CD40L proteins are properly folded. (A) Schematic of the Env, Env-APRIL, Env-BAFF, and Env-CD40L proteins. The clade B JR-FL gp140 protein (amino acids 31 to 681) contains several modifications for stabilization that have been previously described (see Materials and Methods). Codon-optimized sequences encoding the active domains of human and rabbit APRIL, BAFF, or CD40L were added to the gp140 open reading frame at the C terminus. (B) Reducing SDS-PAGE and (C) BN-PAGE analysis of Env, Env-APRIL, Env-BAFF, and Env-CD40L proteins secreted from transiently transfected 293T cells. Recombinant purified JR-FL gp120 (50 ng) and Env from a control transfection were included for comparison. (D) Recognition of Env-APRIL and Env-BAFF by MAbs and CD4. The Env-APRIL and Env-BAFF proteins were immunoprecipitated by CD4-IgG2 and HIVIg MAbs to gp120 (b12) or gp41 (2F5) or via a CD4-induced epitope (MAb 17b) on gp120 in the absence or presence of soluble CD4 (sCD4), followed by reducing SDS-PAGE and Western blot analysis.