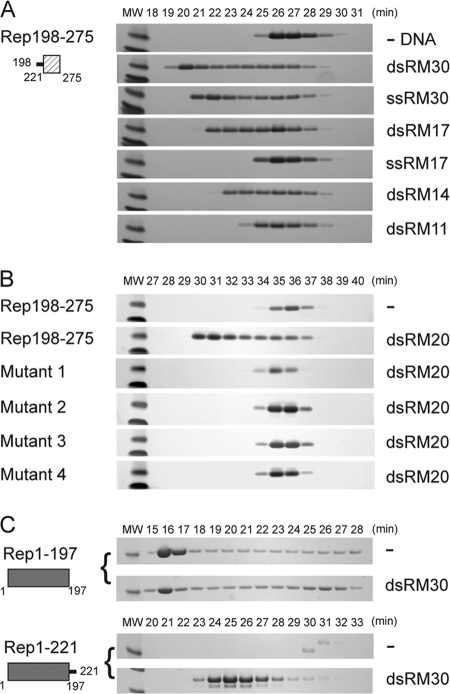
Fig 3.
The linker region confers DNA binding to the small helical bundle of the Rep helicase. (A) Rep198-275 (2.2 mg/ml; 235 μM) was mixed with the indicated oligonucleotides at a 6:2 molar ratio and dialyzed into binding buffer as described in the text. Fractions eluting during SEC on a Superdex 75 column were analyzed by SDS-PAGE, and the numbers correspond to elution times in minutes. The two molecular weight (MW) markers at the left of each gel are 6,000 (top) and 3,500 (bottom). (B) Similarly, the binding of dsRM20 by wild-type Rep198-275, mutant 1 (K213A/K215A/K219A), mutant 2 (P210A/V211A/I212A/K213A), mutant 3 (S214A/K215A/T216A/S217A), and mutant 4 (K213A/S214A/K215A) was assessed on a Superdex 200 column. The protein concentrations used were 300 to 400 μM (between 3 to 4 mg/ml), except for mutant 1, where the concentration was only 110 μM, due to limited recovery of the monomeric form. (C) Binding of dsRM30 by Rep1-197 and Rep1-221. Rep1-197 (205 μM) and Rep1-221 (215 μM) were mixed with dsRM30 at 6:2 molar ratio and dialyzed in binding buffer prior to SEC on a Superdex 200 column. The molecular weight (MW) marker on the left of each SDS-PAGE gel corresponds to Mr 21,000. The numbers above the lanes correspond to elution times in minutes.