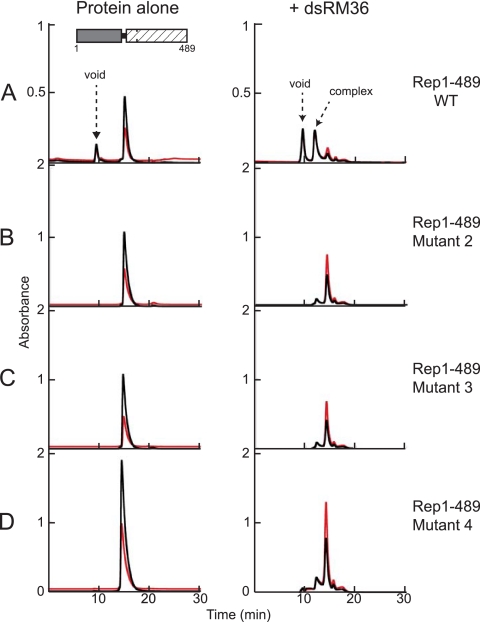
Fig 4.
Mutations in the linker region prevent nonspecific dsDNA binding by Rep1-489. Proteins were mixed with dsSO36 at a 6:2 molar ratio and dialyzed in binding buffer as described in the text. Complexes were subsequently analyzed using an Agilent SEC-5 column at a flow rate of 0.2 ml/min. Samples without DNA (left) were run in 0.5 M NaCl-containing buffer, and the samples with DNA (right) in 50 mM NaCl binding buffer. Prior to dialysis, the concentration of the wild-type Rep1-489 was 2.5 mg/ml, and the concentrations of the mutants were all 4 to 5 mg/ml. The A260 is indicated in red; the A280 is indicated in black. (A) Rep1-489. (B) Rep1-489 mutant 2 (P210A/V211A/I212A/K213A). (C) Rep1-489 mutant 3 (S214A/K215A/T216A/S217A). (D) Rep1-489 mutant 4 (K213A/S214A/K215A). After dialysis, light precipitate was observed in the mixture of Rep1-489 and dsSO36, and very heavy precipitate was observed with the mutants, a finding similar to that observed for the mutant proteins in binding buffer in the absence of DNA.