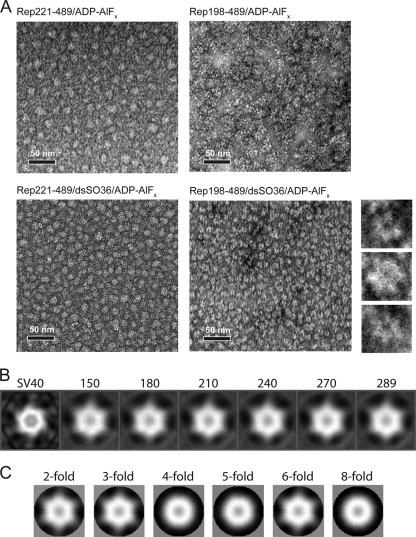
Fig 6.
Rep198-489 bound to nonspecific dsDNA forms hexameric assemblies in the presence of ADP-AlFx. (A) Negatively stained electron micrographs of Rep complexes. Images show Rep221-489 in the presence of ADP-AlFx (top left), Rep198-489 in the presence of ADP-AlFx (top right), Rep221-489 in the presence of dsSO36 DNA and ADP-AlFx (bottom left), and Rep bound to dsSO36 DNA in the presence of ADP-AlFx (bottom right). The smaller images to the right show three representative close-up images of Rep198-489/dsSO36/ADP-AlFx complexes that illustrate the apparent 6-fold symmetry. Scale bars, 50 nm. (B) Reference-based classification and 2D averaging of individual Rep198-489/dsSO36/ADP-AlFx complexes. Individual particles were compared to reference projections derived from the helicase hexamer of the SV40 large T antigen (EMD-1648) (labeled SV40 on right) and ranked according to cross-correlation values. Averages calculated from the top 150, 180, 210, 240, 270, and 289 particles in the data set with the highest cross-correlation values are shown. Note the hexameric appearance in the absence of externally applied symmetry constraints. (C) 2D averages retain structural features only when 2-, 3-, or 6-fold symmetry is applied.