Fig 8.
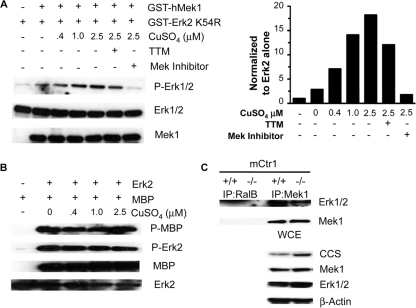
Mek1 kinase activity and association with Erk are stimulated by Cu. (A) Recombinant, GST-tagged human kinase-dead Erk2 and recombinant, GST-tagged human Mek1 were incubated with increasing amounts of CuSO4 with or without TTM or Mek inhibitor 1. Mek1 phosphorylation of Erk2 was assessed by Western blotting with Erk1/2 phosphospecific antibody (n = 3). (B) Erk kinase activity in not enhanced by the addition of Cu. Recombinant GST-hErk2 and recombinant MBP were incubated with increasing amounts of CuSO4. Erk2 phosphorylation of MBP was assessed by Western blotting with MBP phosphospecific antibody (n = 2). (C) Coimmunoprecipitation of Mek1 and Erk1/2 in Ctr1+/+ and Ctr1−/− MEFs. Mek1 immunoprecipitation (IP) from Ctr1+/+ and Ctr1−/− and binding of Erk1/2 were assessed by Western blotting with Mek1 and Erk1/2 antibodies. RalB immunoprecipitation was used as a negative control. Cu deficiency was assessed by immunoblot analysis of CCS protein levels in whole-cell extract (WCE). Immunoblot analyses of total Mek1, Erk1/2, and β-tubulin served as loading controls (n = 3).