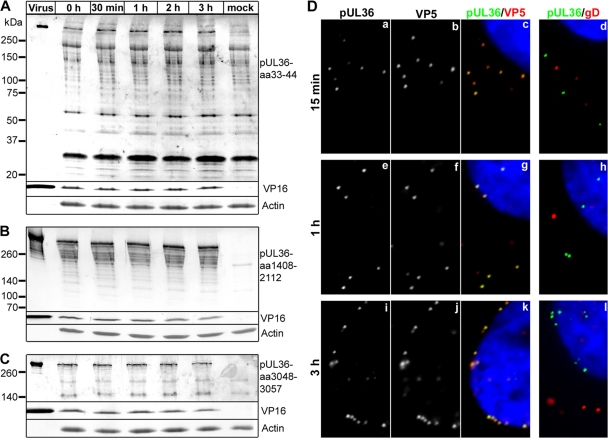
Fig 5.
Full-length pUL36 remains associated with parental, incoming HSV1 capsids. (A to C) Vero cells were either infected with HSV1(17+) at an MOI of 200 PFU/cell in the presence of cycloheximide and harvested at the indicated time points or mock treated for 3 h (mock). Additionally, gradient-purified HSV1(17+) secreted from infected BHK cells and purified on a linear Nycodenz gradient (virus) was loaded. Proteins were separated on linear 5 to 15% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with antibodies directed against pUL36 aa 33 to 44 (A; Nterm, pAb R28), pUL36 aa 1408 to 2112 (B; middle, pAb 146,), pUL36 aa 3048-3057 (C; Cterm, pAb R29), VP16 (MAb LP1), or actin (MAb 1501). (D) Vero cells were infected with HSV1(17+) at an MOI of 50 PFU/cell in the presence of cycloheximide and fixed at the indicated time points with 3% paraformaldehyde, followed by TX-100 permeabilization. The cells were labeled with antibodies directed against pUL36 aa 1408 to 2112 (green and a, e, and i; middle, pAb 147), the major capsid protein VP5 (b, f, and j and red in c, g, and k; MAb 5C10), or the viral membrane protein gD (red in d, h, and l; MAb DL-6) and the nuclei (Hoechst dye; blue in c, d, g, h, k, and l) and analyzed by epifluorescence microscopy.