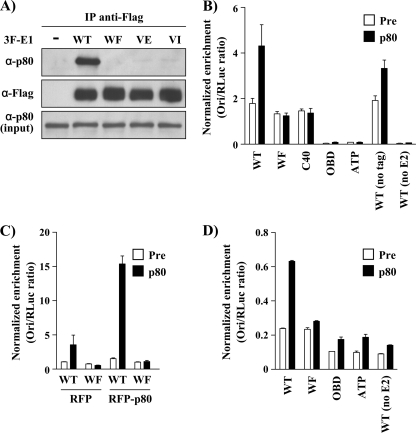
Fig 5.
Endogenous p80 interacts with E1 and is recruited to the viral origin in vivo. (A) Coimmunoprecipitation of endogenous p80 with WT or p80-binding-defective 3F-E1 (WF, VE, and VI). C33A cells were transfected with the indicated E1 expression vectors and harvested 48 h posttransfection, and whole-cell extracts were subjected to immunoprecipitation using an anti-Flag antibody. The immunoprecipitates were analyzed using an anti-Flag antibody or a p80 antiserum. (B) ChIP assays were performed in C33A cells cotransfected with plasmids encoding 3F-E1 (WT or mutant) and RFP-E2 and one containing the origin (pFLORI31). Equal quantities of cell lysates were immunoprecipitated with a rabbit antiserum raised against p80 or the preimmune serum (Pre). (C) ChIP assays were repeated with WT and WF E1 under conditions similar to those described for panel B but in the presence of overexpressed RFP-p80. (D) C33A cells were transfected as described for panel B but were treated with 5 μg/ml of aphidicolin 4 h posttransfection. ChIP assays were performed on the lysates using a p80 antiserum or preimmune serum. For all ChIP experiments, the results of the ori enrichment levels determined by qPCR are shown after normalization to input DNA, using the internal control pRL (RLuc). Each value is the average of at least two replicates, with the standard deviations presented as error bars.