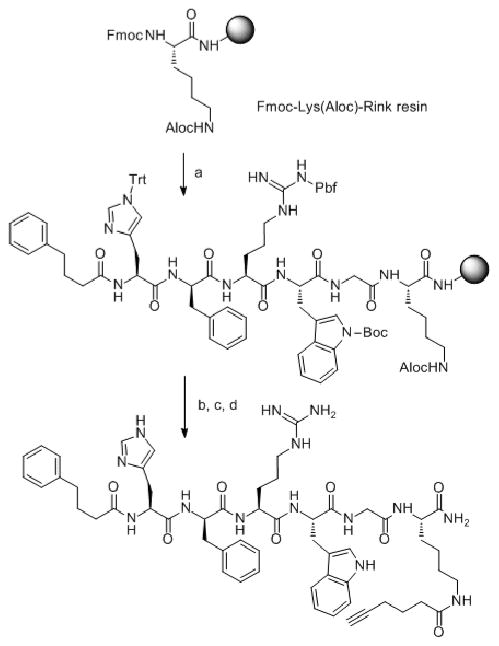
Scheme 1.
Synthetic route for compounds 1–8. a) (i) Fmoc-AA-OH (3eq), HOCt or HOBt (3eq), and DIC (3eq) in DMF/DCM (10 mL/1g of resin) for amino acid couplings; (iii) Piperidine/DMF (1:10, 2 + 20 minutes); (iv) 4-phenylbutyric acid (6eq), and DIC (3eq) in DMF/DCM; b) (i) Pd(0)tetrakistriphenylphosphine (0.01eq), N,N′-dimethylbarbituric acid (5eq) in degassed DCM (2 × 30 minutes) (ii) 5-hexynoic acid (5eq) and DIC (3eq) in DMF/DCM for compound 1; S-Trt-3-propanoic acid (5eq) and DIC (3eq) in DMF/DCM for compound 2; c) (i) TFA-scavengers cocktail (91% TFA, 3% water, 3% thioanisole, 3% ethanedithiol); (ii) ether extraction; d) purification.