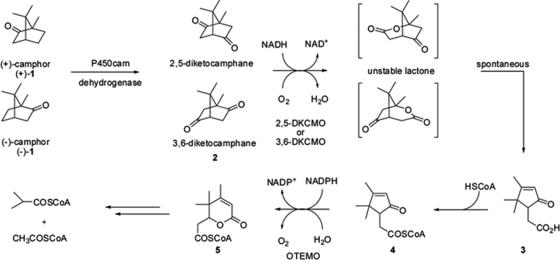
Fig 1.
Catabolic steps of conversion of camphor isomers to acetyl-CoA and isobutyryl-CoA in Pseudomonas putida ATCC 17453. A cytochrome P450-containing enzyme complex (CamCAB) hydroxylates (+)- and (−)-camphor at the 5-exo position to produce 5-exo-hydroxycamphor; upon dehydrogenation (5-exo-hydroxycamphor dehydrogenase [CamD]), the respective diketocamphane is formed. Ring oxygen insertion by the FMN- and NADH-dependent 2,5-diketocamphane monooxygenase for (+)-camphor or 3,6-diketocamphane monooxygenase for (−)-camphor (type 2 BVMOs) produces an unstable lactone that presumably undergoes spontaneous hydrolysis to form 2-oxo-Δ3-4,5,5-trimethylcyclopentenylacetic acid (compound 3). The activation of compound 3 by a putative CoA synthetase produces 2-oxo-Δ3-4,5,5-trimethylcyclopentenylacetyl-CoA, a substrate for OTEMO (type 1 BVMO), the subject of this study. Cumulative data are from references 30, 44, and 58. COSCoA, carbonyl-CoA; HSCoA, acetyl-CoA.