Figure 1.
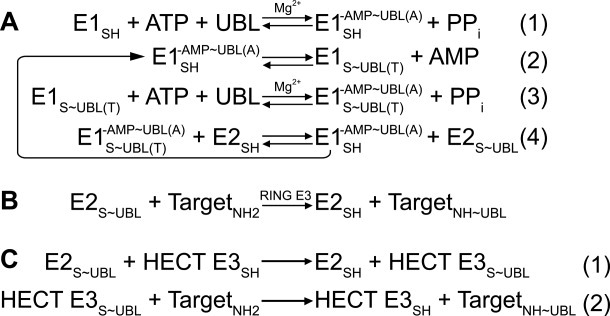
Generalized enzymatic mechanisms of UBL transfer between enzymes and ultimately to a target, based on studies of ubiquitin. ∼ refers to covalent complex; - refers to noncovalent complex. A, Initial steps catalyzed by E1. (1) E1 binds MgATP and a UBL, and catalyzes acyl-adenylation of the UBL's C-terminus. (2) E1 catalytic cysteine attacks the UBL∼AMP intermediate, to form the covalent thioester-linked E1∼UBL intermediate. (3) E1 repeats adenylation reaction on a 2nd UBL molecule, such that E1 binds 2 UBL molecules: UBL(T) is thioester-linked to E1's catalytic cystine; UBL(A) is associated noncovalently at the adenylation site. (4) Doubly-UBL-loaded E1 binds an E2. UBL(T) is transferred from the E1 cysteine to the E2 catalytic cysteine. B, RING E3s (ca. 600 in humans) enhance UBL transfer from E2 to a target. C, HECT E3s (almost 30 in humans) contain a catalytic cysteine, and (1) form a covalent thioester intermediate with a UBL prior to UBL ligation to a target lysine (2). Adapted from Ref. 38.
