Figure 4.
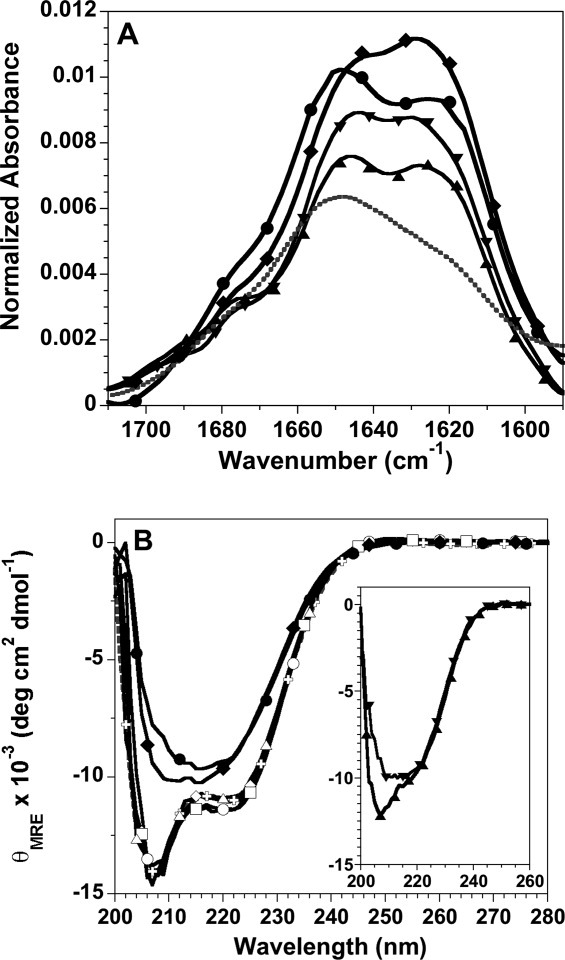
Spectroscopic characterization of histone fibrils. A: FTIR spectra of fibrils formed at pH 5: H3 fibrils, upward triangle; H4 fibrils, downward triangle; fibrils initiated from unfolded H3 + H4, diamonds; fibrils initiated from folded tetramer, circles. For comparison, the spectrum of folded tetramer at pH7.2 is shown as a gray, dotted line. The buffer background spectrum has been subtracted from the protein spectra. B: Far-UV CD spectra normalized to mean residue ellipticity: tetramer at pH 7.2, gray crosses and dotted line; tetramer at pH 5, t = 0 and 196 h, open and closed circles, respectively; initially unfolded H3 + H4 at pH 5, t = 0 and 196 h, open and closed diamonds, respectively; t = 0 spectra for tetramer and H3 + H4 at pH 2, open squares and triangles, respectively. Inset: t = 0 spectra for the individual H3 and H4 monomers, upward and downward triangles, respectively. Fibrillation conditions: 1M NaCl, 50 mM acetic acid/sodium acetate and 50 mM phosphoric acid/NaH2PO4, pH 5 or pH 2, 23°C and 30 μM of the individual H3 and H4 monomers or 30 μM of each monomer for tetramer samples and H3 + H4 initially unfolded in 10 mM HCl.
