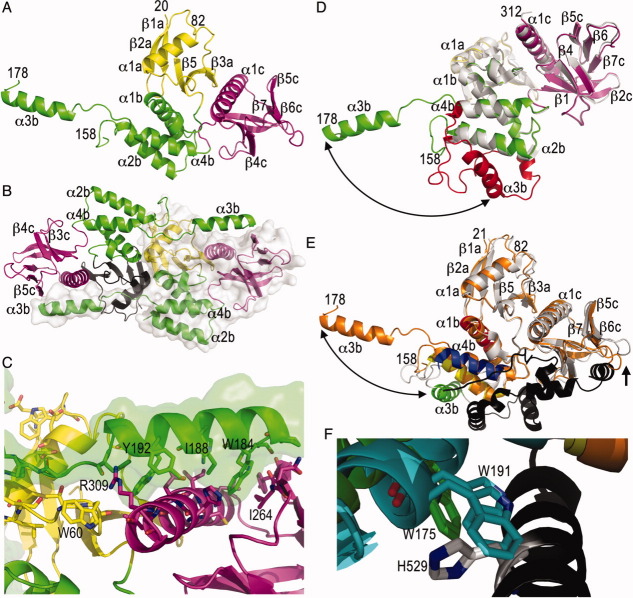
Figure 1.
The merlin FERM domain structure is unfurled. (A) Cartoon drawing of the human merlin head FERM domain. The F1 subdomain (residues 20–82 and 91–100) is shown in yellow, the F2 subdomain (residues 101–158 and 178–215) is shown in green, and the F3 motif (residues 216–313) is shown in magenta. Some termini (21, 82, 158, and 178) and secondary structure elements (“a” belonging to the F1, “b” to F2, and “c” to the F3 subdomains) are labeled in several panels. (B) The unfurled F2 subdomain engages in additional contacts with another monomer, which is shown as a surface representation. The FERM subdomains are colored as in panel (A) (F1, yellow or black; F2, green; and F3, magenta). (C) Detailed view of the intermolecular interactions of the extended F2 α3b α-helix (F2, green) with a two-fold related molecule (F1, yellow; and F3, magenta). A surface representation is also shown for the F2 subdomain. (D) Superposition of our unfurled merlin head domain (molecule “C”; F1, yellow; F2, green; and F3, magenta) onto the closed, unbound FERM domain structure of merlin (PDB entry 1h4r; white and red) is shown. The two molecules in the closed FERM structure superimpose with r.m.s.d. of 1.3 and 1.4 Å for 1965 atoms of our unfurled merlin structure. The large movement of the α-helix α3b of the F2 subdomain (red) is indicated by the arrow. (E) Superposition of the unfurled merlin structure (molecule “C,” orange) onto the moesin head:tail complex structure (PDB entry 1ef1; F1 and F2, white; F2 α-helix α1b, moesin residues 95–112, red; F2 α2b α-helix, moesin residues 118–135, yellow; F2 α3b α-helix, moesin residues 164–179, green; F2 α-helix α4b, moesin residues 183–196, blue; tail, black) with r.m.s.d. of 1.9 Å for 1780 atoms of the two moesin FERM domains in the asymmetric unit. The large movement of α-helix α3b is indicated by a double arrow. The movement of the β6c-β7c loop that seems necessary to allow tail binding is indicated by an arrow. (F) Close-up view of the movement of the α-helix α3b upon tail binding. Trp191 residing on the F2 α-helix α3b of the superimposed closed, unbound merlin FERM conformation clashes with the tail domain, in particular with His529.