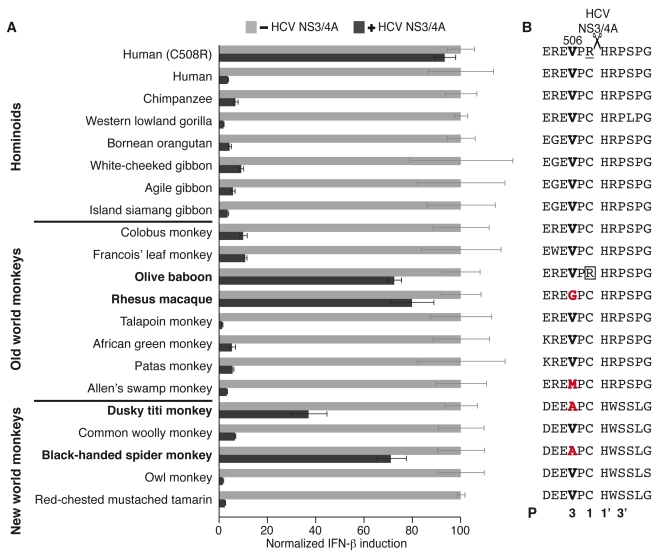
Figure 2. MAVS from multiple primate species is resistant to antagonism by HCV protease NS3/4A.
(A) Induction of IFN-β promoter, as measured by luciferase firefly activity, upon expression of MAVS cDNA from corresponding species coexpressed with (+) or without (−) HCV NS3/4A. Primates with MAVS capable of significant IFN-β induction even in the presence of HCV protease are highlighted in bold. Human (C508R) refers to substitution of Cysteine (C) at position 508 with Arginine (R) in human MAVS. Firefly luciferase activity is normalized as being 100% for MAVS from each species in absence of HCV NS3/4A. All experiments are done in triplicates, and error bars indicate standard deviation. (B) Amino acid sequence of MAVS (residues 503–514) from corresponding primates in (A). The scissor icon indicates HCV NS3/4A cleavage site. The P positions for the HCV protease are indicated at the bottom of the alignment. Arginine (R) at residue 508 in olive baboon, believed to prevent MAVS cleavage, is boxed and the C508R change in human MAVS is underlined. Position 506, highlighted in bold, correlates with the ability of primate MAVS to induce IFN-β even in the presence of HCV NS3/4A protease as seen in (A). Changes away from the ancestral valine (V) at position 506 are indicated in red.