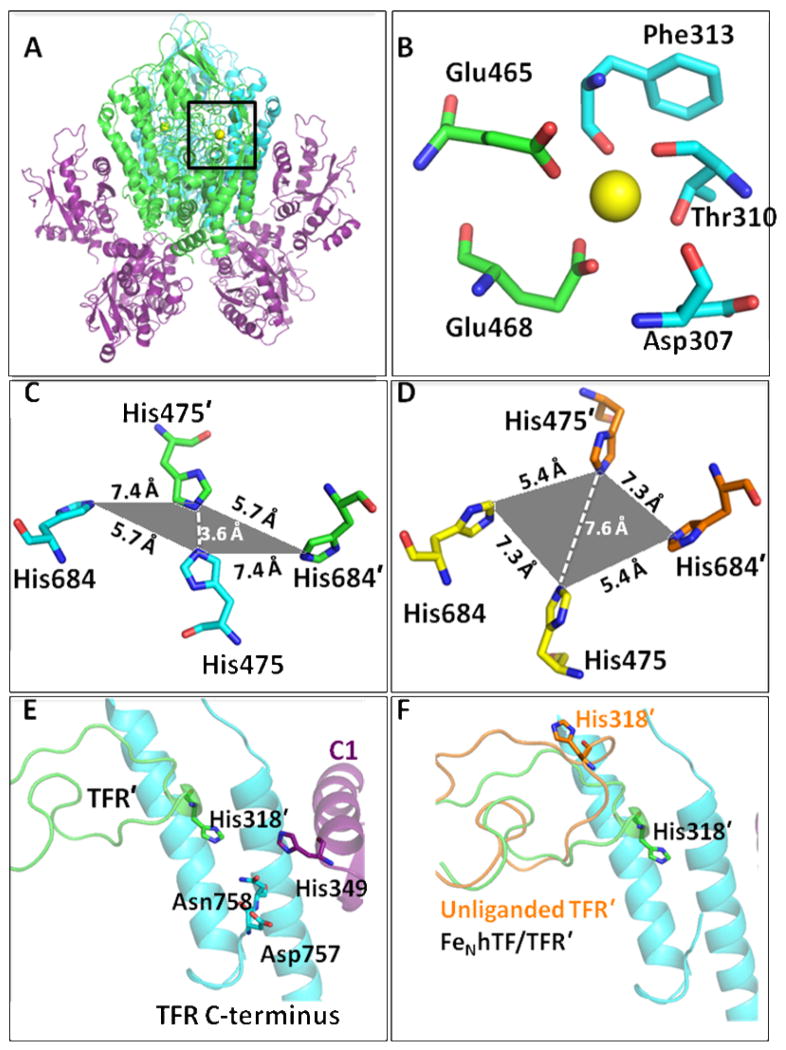
Figure 1.
(A) The FeNhTF/sTFR crystal structure (PDB ID: 3S9L).15 One TFR monomer (TFR) is shown in cyan, the other TFR monomer (TFR′) is shown in green and the two molecules of FeNhTF are shown in purple. (B) Ca2+ binding site located between the protease-like (green) and apical domains (blue) of the TFR (A, black box). (C) The histidine cluster formed at the dimer interface. Two His residues, His475 and His684, from one TFR monomer (cyan) converge with the same two His residues, His475′ and His684′, from the other TFR monomer (green) as a result of hTF binding (in comparison to their position in the unliganded TFR structure, PDB ID: 1CX8 13 as shown in yellow and orange in (D)). (E) The TFR-TFR′-C1 intersection formed when hTF binds to the sTFR. One TFR monomer is shown in cyan, the other TFR monomer (TFR′) is shown in green and the FeNhTF is shown in purple. (F) The long loop containing His318 moves ~18 Å upon hTF binding, as shown by overlaying the structure of the unliganded TFR (orange) with the sTFR in the FeNhTF/sTFR structure (green).