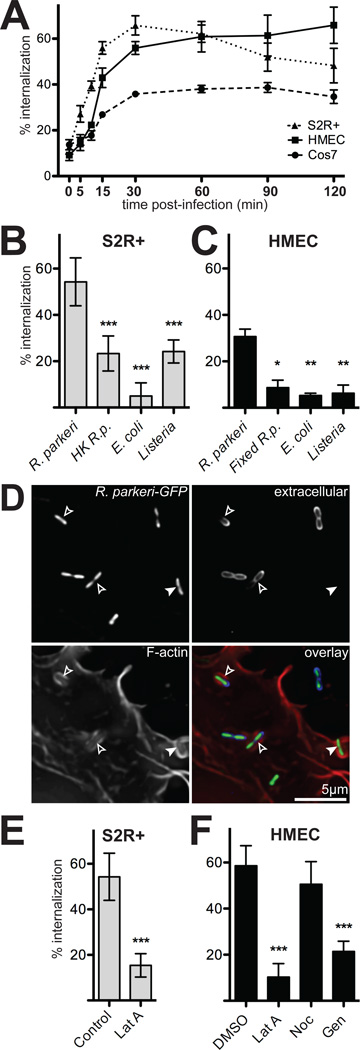
Figure 1. R. parkeri invade cells quickly in a process dependent on viable bacteria and host actin.
(A) Percent of cell-associated R. parkeri that were internalized by S2R+ (triangles), HMEC-1 (squares) or COS-7 (circles) cells at various times post-infection (n=3). (B) Percent internalization by S2R+ cells infected for 15 min with live R. parkeri, heat-killed R. parkeri (HK R.p.), E. coli XL-10 Gold (E. coli), or L. monocytogenes 10403S (Listeria). (C) Percent internalization by HMEC-1 infected for 15 min with live R. parkeri, or formaldehyde-fixed R. parkeri (Fixed R.p.), L. monocytogenes, or E. coli. Note: live R.p. in panel C were incubated at RT while samples were fixed (D) Maximum intensity projection of a deconvolved image of COS-7 cells transfected with pLifeact-mCherry (lower left, red in merge) and infected 48 h later with GFP-expressing R. parkeri (GFP, top left, green in merge) for 15 min. Extracellular bacteria were stained with anti-Rickettsia antibody without permeabilization (upper right, blue in merge). Open arrowheads; partially internalized bacteria, closed arrowheads; fully internalized bacterium. (E) Internalization by S2R+ cells infected for 15 min with R. parkeri and untreated (Control) or treated for 30 min prior to infection with 4 µM latrunculin A (Lat A). (F) Internalization by HMEC-1 cells after 15 min infection; cells were either pretreated with 1% DMSO alone, or treated 30 minutes before infection with 4 µM latrunculin A (LatA), 20 µM nocodazole (Noc) or 250 µM genistein (Gen) in 1% DMSO. For all panels: graphs plot the mean percent internalization for at least three independent experiments. Error bars indicate SEM (A), SD (B–D); * p<0.05, ** p<0.01, *** p<0.001 versus mean of control (R. parkeri or DMSO) by unpaired t-test.