Abstract
There are several data concerning transporters expression and/or regulation in cell lines maintained in different conditions, such as medium glucose concentration. This work aimed to evaluate the influence of two different extracellular glucose concentrations, commonly used in culture media, on the intestinal absorption of organic cations. Thus, the effect of 5.5 mM glucose and 25 mM glucose (HG) in culture media, was studied on [3H]-MPP+ (1-methyl-4-phenylpyridinium iodide) uptake in Caco-2 cells. Expression of human organic cation transporter type 1 (hOCT1) and human organic cation transporter type 3 (hOCT3) was investigated in cells cultured at both glucose concentrations. [3H]-MPP+ uptake, as well as its affinity for the transporter, were significantly decreased in HG cells. Moreover, hOCT3 mRNA levels were reduced in HG cells. Functional confirmation of this result was made using hOCT3 inhibitors. In conclusion, maintenance of Caco-2 cells (commonly used in several in vitro studies on membrane transport) in HG conditions affects organic cation transport at the intestinal level. Hence, results obtained in these conditions must be analysed with great care, since extracellular glucose levels may originate changes in organic cation nutrient and drug bioavailability.
Keywords: Absorption, Caco-2 cells, Glucose, Intestinal epithelium, Organic cations, Transport
Introduction
It has been well established that high glucose environments favour oxidative stress through mechanisms that include nonenzymatic, enzymatic and mitochondrial pathways. As a consequence, cellular components (lipids, proteins and nucleic acids) may undergo significant modifications that can culminate in cell injury. Several reports focusing on aging and diabetes mellitus have implicated high extracellular glucose concentrations in significant alterations in regulatory and functional activity of proteins (Cornford et al. 1995; Giannico et al. 2007; Ha and Lee 2000; Hahn et al. 1998; Noyman et al. 2002; Stevens et al. 1999). If transporters that regulate molecule transfer in exchange surfaces (like the intestinal epithelium) are targets of such glucose-induced alterations, bioavailability of their substrates can be compromised.
Hence, this work was performed in order to evaluate the influence of long-term exposure of Caco-2 cells to high extracellular glucose concentrations on the functional activity of intestinal organic cation transporters. Organic cations are polar and positively charged compounds at physiological pH, and so membrane-bound transport systems are necessary for the absorption, distribution, and elimination of these compounds. Therefore, intestinal transporters play a crucial role in limiting and/or promoting the absorption or secretion of organic cations (Hardman et al. 2002). The class of organic cations includes numerous therapeutic drugs, but also endogenous bioactive amines, xenobiotics and nutrients.
Caco-2 cells are extensively used as an enterocyte cell model in physiological studies (Artursson 1991; Artursson and Karlsson 1991; Delie and Rubas 1997; Hidalgo et al. 1989; Lennernas 1997; Muller et al. 2005; Yee 1997). Different culture conditions, such as seeding density or medium composition, are recognized as important culture-related factors, being able to modify cell phenotype (Sambuy et al. 2005). Thus, it is difficult to discuss results obtained with Caco-2 cells from different labs, as they are maintained in different culture conditions. In relation to glucose concentration, there are several published data concerning OCTs expression and/or regulation in cell lines maintained in MEM [5.5 mM glucose; (Martel et al. 2000)] or DMEM [25 mM glucose; (Hayer-Zillgen et al. 2002; Kimura et al. 2009)], but the influence of medium glucose concentration on organic cation transport remained unexplored.
Thus, the present work aim to investigate the transport of the model organic cation [3H]-MPP+ in Caco-2 cells maintained under physiological (5.5 mM) or high (25 mM) glucose concentrations. According to the literature (Martel et al. 2001), organic cation transporter 1 (hOCT1) and organic cation transporter 3 (hOCT3 or hEMT) are the transporters implicated in organic cation uptake at the brush-border membrane of Caco-2 cells. So, investigation of mRNA expression levels of these two [3H]-MPP+ transporters was also performed for both culture conditions.
Materials and methods
Materials
[3H]-MPP+ (N-[methyl-3H]-4-phenylpyridinium acetate; specific activity 82 Ci mmol−1; New England Nuclear Chemicals, Dreieich, Germany); Triton X-100 and d-(+)-glucose (Merck, Darmstadt, Germany); MPP+ (1-methyl-4-phenylpyridinium iodide) clonidine and corticosterone (Sigma, St. Louis, MO, USA).
Cells and culture conditions
Caco-2 cell line was obtained from the American Type Culture Collection (ATCC37-HTB, Rockville, M.D., U.S.A.) and was used between passage number 30–41. Caco-2 cells were maintained in a humidified atmosphere of 5% CO2–95% air and were grown in Minimum Essential Medium (Sigma, St. Louis, M.O., U.S.A.) containing either 25 mM (high) or 5.5 mM (physiological) glucose and supplemented with 15% fetal bovine serum, 25 mM HEPES, 100 units mL−1 penicillin, 100 μg mL−1 streptomycin and 0.25 μg mL−1 amphotericin B (all from Sigma). Caco-2 cells were adapted for at least five passages to the high glucose concentration. Osmolarity control was performed with mannitol. Culture medium was changed every 2–3 days and the culture was split every 7 days. For subculturing, the cells were removed enzymatically (0.25% trypsin–EDTA), split 1:3, and subcultured in plastic culture dishes (21 cm2; ∅ 60 mm; Corning Costar, Corning, N.Y.). For the experiments, Caco-2 cells were seeded on 24-well plastic cell culture clusters (2 cm2; ∅ 16 mm; Corning Costar). Uptake studies were performed 9–11 days after the cells formed a monolayer (confluence).
Transport studies
Transport experiments were performed in Hanks’ medium with the following composition (in mM): 137 NaCl, 5 KCl, 0.8 MgSO4, 1.0 MgCl2, 0.33 Na2HPO4, 0.44 KH2PO4, 0.25 CaCl2, 0.15 tris.HCl, and 1.0 sodium butyrate, pH 7.4.
Transport studies were performed in cells cultured on plastic supports, [3H]-MPP+ being applied to the medium facing the apical cell membrane. Initially, the growth medium was aspirated and the cells were washed with Hanks’ medium at 37 °C; then the cell monolayers were preincubated in Hanks’ medium at 37 °C. Uptake was then initiated by the addition of 0.3 mL medium at 37 °C containing 200 nM [3H]-MPP+. Incubation was stopped after 5 min by placing the cells on ice and rinsing them with 0.5 mL ice-cold Hanks’ medium. The cells were then solubilized with 0.3 mL of 0.1% (v/v) triton X-100 (in 5 mM tris.HCl, pH 7.4), at room temperature overnight. Radioactivity in the cells was measured by liquid scintillation counting. In kinetic experiments, cells were preincubated for 60 min with Hanks’ medium and incubated with increasing concentrations of [3H]-MPP+ (0.2–500 μM) for 5 min.
Compounds to be tested (corticosterone and clonidine) were present during both the preincubation and incubation periods. Controls (ethanol) for these treatments were run in the presence of the solvent (1%).
RNA extraction and RT–PCR
RNA was extracted from the cells, from three independent cultures, using Tripure Isolation Reagent (Roche, Indianapolis, USA), according to the producer’s instructions. RNA was dissolved in water (diethylpyrocarbonate-treated) and stored at −80 °C.
Five μg of RNA were used as template for cDNA production through incubation with reverse transcriptase (Reverase, Bioron GmbH) for 1 h at 45 °C, in 10 μM random hexamers, 0.375 mM per dNTP, 3 mM MgCl2, 75 mM KCl, 50 mM Tris–HCl, pH 8.3, 10 mM dithiothreitol, and 40 units RNase inhibitor (RNaseOUTTM; Gibco BRL), followed by 10 min at 95 °C to inactivate the enzyme. Samples were incubated for 30 min at 37 °C with 0.1 mg/mL RNAse (Sigma). PCR amplification was performed in the presence of 2 mM of MgCl2, 0.5 mM of primer, 0.2 mM dNTPs, 2 U of Taq DNA polymerase (DFS-Taq DNA polymerase, Bioron GmbH) and 4 μL of RT product, in a final volume of 50 μL. Simultaneous amplification of the invariant housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed.
The following primers, for human organic cation transporter 3 (hOCT3), human organic cation transporter 1 (hOCT1) and GAPDH were used: 5′-CAC ACT TAT TCT TAT GTT TGC TTG-3′ (forward primer hOCT3), 5′-GAT AGC TCC TTC TTT CTG TCT TTG-3′ (reverse primer hOCT3), 5′-TGG GAA GTT GCC TCC TGC TGA TT-3′ (forward primer hOCT1), 5′- CAG ACC TCC CTC AGC CTG AAG ACT AT-3′ (reverse primer hOCT1), 5′-ACT GGC GTC TTC ACC ACC AT-3′ (forward primer GAPDH), 5′-TCC ACC ACC CTG TTG CTG TA-3′(reverse primer GAPDH; primers from Metabion International—Martinsried, Deutschland). Thermocycling consisted of 34 cycles of 30 s at 94 °C, 1 min at 54 °C (hOCT1) or 55 °C (hOCT3 and GAPDH) and 1 min at 72 °C. The predicted sizes of the PCR products were (in bp): 550 (hOCT1), 654 (hOCT3) and 682 (GAPDH). PCR products were visualized on a 1.6% agarose gel with ethidium bromide staining. The expression of all tested enzymes was normalized to the expression of GAPDH of each sample and compared using Gel Pro Analyser® software.
Protein determination
The protein content of cell monolayers was determined using Bradford methodology as described (Bradford 1976), with human serum albumin as standard.
Kinetic studies
Kinetic parameters were determined by non-linear regression analysis using the following equation: 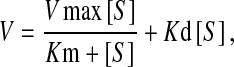 where V is the velocity of the uptake, Km is the substrate binding affinity or Michaelis–Menten constant, Vmax is the maximum transport capacity, [S] is the concentration of 3H-MPP+ and Kd is the diffusion coefficient. Analysis was made using Graph Pad Prism Software version 3.02.
where V is the velocity of the uptake, Km is the substrate binding affinity or Michaelis–Menten constant, Vmax is the maximum transport capacity, [S] is the concentration of 3H-MPP+ and Kd is the diffusion coefficient. Analysis was made using Graph Pad Prism Software version 3.02.
Statistics
Values are expressed as the arithmetic mean ± SEM. Statistical significance of the difference between various groups was evaluated by one-way analysis variance (ANOVA) followed by the Bonferroni test. For comparison between two groups, Student’s t-test was used. Differences were considered to be significant when P < 0.05.
Results
As previously reported (Martel et al. 2000), uptake of [3H]-MPP+ into Caco-2 cells is linear in time for up to 5 min of incubation. Therefore, in order to determine initial rates of uptake, cells were incubated in the presence of [3H]-MPP+ (200 nM) for 5 min.
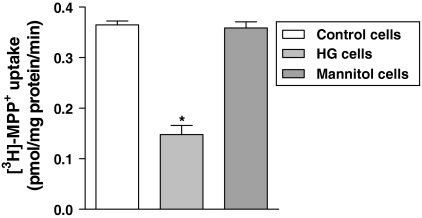
Caco-2 cells cultured under physiologic (control cells) and high glucose (HG cells) concentrations were preincubated for 60 min with Hanks’ buffer and incubated with [3H]-MPP+ (200 nM) for 5 min. [3H]-MPP+ uptake was found to be significantly reduced in HG cells (0.16 ± 0.0 pmol/mg protein/min; n = 6) relatively to control cells (0.36 ± 0.01 pmol/mg protein/min; n = 6; Fig. 1).
Fig. 1.
The inhibitory effect on [3H]-MPP+ apical uptake by Caco-2 cells cultured in high glucose (HG cells) versus cultured under 5.5 mM (control cells). Osmolarity control was performed with mannitol. Confluent Caco-2 monolayers were preincubated at 37 °C for 60 min with Hanks’ buffer and then incubated with 200 nM [3H]-MPP+ for 5 min. Each value represents the mean ± SEM of the triplicates of two independent experiments (n = 6). * P < 0.05 in comparison to control cells
The concentration dependency of the initial uptake rates was next studied by incubating Caco-2 cells with increasing concentrations (0.2–500 μM) of [3H]-MPP+, applied from the apical cell membrane, for 5 min. The kinetic features of [3H]-MPP+ uptake for both cultures of cells were examined. A significant increase was observed in Km in HG cells (43.9 ± 2.0 μM in control vs. 88.1 ± 4.2 μM in HG cells) but no significant changes were found in the respective Vmax values (0.30 ± 0.01 vs. 0.28 ± 0.01 nmol/mg protein/min, respectively; Table 1). Apparently passive diffusion (Kd) of [3H]-MPP+ through the apical membrane was decreased in HG cells (2.6 ± 0.01 vs. 2.0 ± 0.01 pmol/mg protein/min/μM, respectively). There were no significant differences on kinetic parameters of [3H]-MPP+ uptake in cells treated with 25 mM mannitol (data not shown).
Table 1.
[3H]-MPP+ apical transport kinetics across Caco-2 cells under 5.5 mM (control cells) and high glucose (HG cells)
| Km (μM) | Vmax (nmol/mg protein/min) | Kd (pmol/mg protein/min/μM) | |
|---|---|---|---|
| Caco-2 | 43.9 ± 2.0* | 0.30 ± 0.01 | 2.6 ± 0.01* |
| HG Caco-2 | 88.1 ± 4.2* | 0.28 ± 0.01 | 2.0 ± 0.01* |
Substrate binding affinity (Km), maximum transport capacity (Vmax) and diffusion coefficient (Kd) for this substrate were determined by nonlinear regression analysis. Each value represents the mean ± SEM of the triplicates of at least three different experiments (n = 12). The values followed by (*) in each column are significantly different at P < 0.05
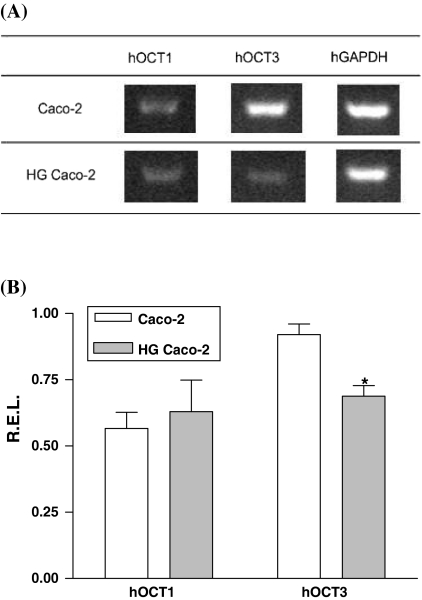
RT–PCR analysis of both hOCT1 and hOCT3 mRNA transcription was performed in control and HG Caco-2 cells. Amplification products were obtained with both hOCT3 and hOCT1 primers. Interestingly enough, quantification of the transcription showed a significant reduction of hOCT3 transcription in HG Caco-2 cells by comparison with control cells (Fig. 2).
Fig. 2.
(A) Representative bands of the effect of extracellular glucose concentration on organic cation transporter 1 (hOCT1) and organic cation transporter 3 (hOCT3) transcriptions. (B) Graphic representation of the quantification of mRNA expression for hOCT1 and hOCT3, normalized by comparison with the expression of GAPDH of each sample (R.E.L.—Relative Expression Level). hOCT3 expression was inhibited in Caco-2 cells cultured in high glucose (HG cells) versus cultured under 5.5 mM (control cells). Each value represents the mean ± SEM of three separate cultures.* P < 0.05
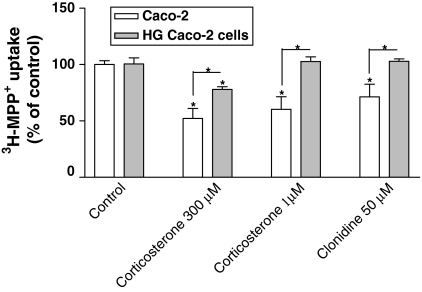
Finally, two inhibitors of OCT-mediated [3H]-MPP+ uptake were also tested in both cultures of cells. As shown in Fig. 3, both compounds inhibited [3H]-MPP+ uptake in control Caco-2 cells. Corticosterone (300 μM) inhibited uptake to 57.5 ± 10.9% of control (control cells) and to 77.9 ± 2.41% of control (HG cells); corticosterone (1 μM) as well as clonidine (50 μM) were also able to inhibite [3H]-MPP+ uptake in control cells, but had no effect on HG cells. These results showed that high glucose levels interfered with the characteristics of [3H]-MPP+ transport, HG cells being less sensitive to the effect of these inhibitors than control cells.
Fig. 3.
Inhibitory effect of corticosterone (1 and 300 μM) and clonidine (50 μM) on [3H]-MPP+ apical uptake by control and HG Caco-2. Confluent Caco-2 monolayers were preincubated at 37 °C in the presence of H2O (control) or the test compounds and then incubated with 200 nM [3H]-MPP+ for 5 min. Each value represents the mean ± SEM of the triplicates of two independent experiments (n = 6). * P < 0.05 (significant increase and decrease)
Discussion
The purpose of this study was to evaluate the influence of different extracellular glucose concentrations on the functional activity of intestinal organic cation absorption.
[3H]-MPP+ uptake was significantly reduced in cells treated with high glucose concentration as compared to control cells. Different mechanisms might have been involved in the observed effect, including modification of substrate or transporter protein as a consequence, for example, of redox alteration in cellular environment. To better understand how glucose affected [3H]-MPP+ transport, saturation experiments were performed with cells cultured under two different glucose concentrations. The maximum transport capacity did not present significant differences between both conditions, although it was slightly decreased in HG Caco-2 cells. On the other hand, in these cells there was a significant increase in Km value, meaning that the affinity of the transporter for [3H]-MPP+ was decreased. According to the literature (Martel et al. 2001), organic cation transporter 1 (hOCT1) and organic cation transporter 3 (hOCT3 or hEMT) are the transporters implicated in organic cation uptake at the brush-border membrane of Caco-2 cells. In order to examine the expression of these two transporters, RT–PCR quantification of mRNA levels for these two transporters was performed. Interestingly, hOCT3 transcription was downregulated in HG cells, whereas hOCT1 mRNA levels were not affected. Functional confirmation of these data was next attempted. Two known inhibitors of [3H]-MPP+ uptake in Caco-2 cells, clonidine and corticosterone (Martel et al. 2001), were tested and showed different effects on [3H]-MPP+ uptake by control and HG cells. When tested in a concentration near the IC50 for control Caco-2 cells (Martel et al. 2001; Muller et al. 2005), clonidine reduced [3H]-MPP+ uptake in control cells, but had no effect on [3H]-MPP+ uptake in HG cells, suggesting that these cells became insensitive to clonidine. Moreover, corticosterone was tested in two concentrations (300 μM and 1 μM) (Martel et al. 2001). Again, HG cells, compared to control cells, presented a loss of responsiveness to corticosterone (this compound was even devoid of effect at 1 μM). The loss of responsiveness to 1 μM corticosterone in HG cells was in good agreement with the observed reduction of hOCT3 expression in these cells, because inhibition of transport by such a low concentration of corticosterone is usually interpreted to reflect inhibition of hOCT3-mediated transport. Altogether, these results suggest that high extracellular glucose concentration affects hOCT3 mRNA transcription and protein activity levels in Caco-2 cells. Similarly, it was previously demonstrated that Caco-2 cells maintained in a 25 mM glucose medium, has a decreased PepT-1 transport activity (D’Souza et al. 2003). According to these authors, an oxidative pathway seems to be responsible for this effect. In top of this, it is known that diabetes also affects the intestinal absorption of several nutrients, e.g. glucose and lipids (Thomson and Wild 1997), amino acids (Casirola et al. 1994; Contreras et al. 1997), dipeptides and tripeptides (Adibi 2003), mannitol (Carratu et al. 1999), vitamin A (Basu and Basualdo 1997), and calcium (Schedl et al. 1995). Also, high glucose levels (the hallmark of diabetes) cause an inhibition of the folate transporter RFC1 (Reduced Folate Transporter 1; Naggar et al. 2002), and of the plasmalemmal serotonin transporter (SERT; Goncalves et al. 2008).
Despite the recent attention given to the role of oxidant state on regulation of biochemical mechanisms in cells (Burgoyne et al. 2007), there is a gap in the literature concerning the importance of the redox state on OCT1- or OCT3-mediated transport, although these transporters possess several amino acid residues in intra- and extracellular domains (Grundemann et al. 1998; Hayer et al. 1999) that are prone to oxidation. Altogether, these results are compatible with a modulation of organic cation transport in Caco-2 cells through redox mechanisms, as has been previously advanced (Faria et al. 2006).
In conclusion, this work’s data support the influence of extracellular glucose concentration upon organic cations transport in intestinal epithelial cells. So, organic cation transport results obtained in studies in which Caco-2 cells were cultured in high (25 mM) glucose medium concentration must be analysed with great care.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (POCI, FEDER, Programa Comunitário de Apoio, PTDC/QUI/65501/2006, SFRH/BD/28160/2006 SFRH/BD/46640/2008 and SFRH/BPD/40110/2007).
Glossary
- MPP+
1-methyl-4-phenylpyridinium iodide
- HG
High glucose
- hOCT1
Human organic cation transporter type 1
- hOCT3
Human organic cation transporter type 3
References
- Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol. 2003;285:G779–G788. doi: 10.1152/ajpgi.00056.2003. [DOI] [PubMed] [Google Scholar]
- Artursson P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit Rev Ther Drug Carrier Syst. 1991;8:305–330. [PubMed] [Google Scholar]
- Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175:880–885. doi: 10.1016/0006-291X(91)91647-U. [DOI] [PubMed] [Google Scholar]
- Basu TK, Basualdo C. Vitamin A homeostasis and diabetes mellitus. Nutrition. 1997;13:804–806. doi: 10.1016/S0899-9007(97)00192-5. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- Carratu R, Secondulfo M, Magistris L, Iafusco D, Urio A, Carbone MG, Pontoni G, Carteni M, Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr. 1999;28:264–269. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- Casirola DM, Vinnakota RR, Ferraris RP. Intestinal amino acid transport in mice is modulated by diabetes and diet. J Nutr. 1994;124:842–852. doi: 10.1093/jn/124.6.842. [DOI] [PubMed] [Google Scholar]
- Contreras R, Fuentes O, Mann GE, Sobrevia L. Diabetes and insulin-induced stimulation of L-arginine transport and nitric oxide synthesis in rabbit isolated gastric glands. J Physiol. 1997;498(Pt 3):787–796. doi: 10.1113/jphysiol.1997.sp021902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Cornford ME, Clare-Salzler M. Down-regulation of blood-brain glucose transport in the hyperglycemic nonobese diabetic mouse. Neurochem Res. 1995;20:869–873. doi: 10.1007/BF00969700. [DOI] [PubMed] [Google Scholar]
- D’Souza VM, Buckley DJ, Buckley AR, Pauletti GM. Extracellular glucose concentration alters functional activity of the intestinal oligopeptide transporter (PepT-1) in Caco-2 cells. J Pharm Sci. 2003;92:594–603. doi: 10.1002/jps.10325. [DOI] [PubMed] [Google Scholar]
- Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst. 1997;14:221–286. [PubMed] [Google Scholar]
- Faria A, Mateus N, Freitas V, Calhau C. Modulation of MPP + uptake by procyanidins in Caco-2 cells: involvement of oxidation/reduction reactions. FEBS Lett. 2006;580:155–160. doi: 10.1016/j.febslet.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Giannico G, Cortes P, Baccora MH, Hassett C, Taube DW, Yee J. Glibenclamide prevents increased extracellular matrix formation induced by high glucose concentration in mesangial cells. Am J Physiol Renal Physiol Physiol. 2007;292:F57–F65. doi: 10.1152/ajprenal.00210.2006. [DOI] [PubMed] [Google Scholar]
- Goncalves P, Araujo JR, Martel F. The effect of high glucose on SERT, the human plasmalemmal serotonin transporter. Nutr Neurosci. 2008;11:244–250. doi: 10.1179/147683008X344156. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int Suppl. 2000;77:S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Weiss U, Mosgoeller W, Desoye G. Sustained hyperglycemia in vitro down-regulates the GLUT1 glucose transport system of cultured human term placental trophoblast: a mechanism to protect fetal development? Faseb J. 1998;12:1221–1231. doi: 10.1096/fasebj.12.12.1221. [DOI] [PubMed] [Google Scholar]
- Hardman J, Limbird L, Molinoff P, Ruddon R, Gilman A. Goodman and Gilman’s, The pharmacological basis of therapeutics. New York: McGraw-Hill; 2002. [Google Scholar]
- Hayer M, Bonisch H, Bruss M. Molecular cloning, functional characterization and genomic organization of four alternatively spliced isoforms of the human organic cation transporter 1 (hOCT1/SLC22A1) Ann Hum Genet. 1999;63:473–482. doi: 10.1046/j.1469-1809.2000.6430267.x. [DOI] [PubMed] [Google Scholar]
- Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- Kimura N, Masuda S, Katsura T, Inui K. Transport of guanidine compounds by human organic cation transporters, hOCT1 and hOCT2. Biochem Pharmacol. 2009;77:1429–1436. doi: 10.1016/j.bcp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Lennernas H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49:627–638. doi: 10.1111/j.2042-7158.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- Martel F, Calhau C, Azevedo I. Characterization of the transport of the organic cation [3H]MPP + in human intestinal epithelial (Caco-2) cells. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:505–513. doi: 10.1007/s002100000223. [DOI] [PubMed] [Google Scholar]
- Martel F, Grundemann D, Calhau C, Schomig E. Apical uptake of organic cations by human intestinal Caco-2 cells: putative involvement of ASF transporters. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:40–49. doi: 10.1007/s002100000335. [DOI] [PubMed] [Google Scholar]
- Muller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Naggar H, Ola MS, Moore P, Huang W, Bridges CC, Ganapathy V, Smith SB. Downregulation of reduced-folate transporter by glucose in cultured RPE cells and in RPE of diabetic mice. Invest Ophthalmol Vis Sci. 2002;43:556–563. [PMC free article] [PubMed] [Google Scholar]
- Noyman I, Marikovsky M, Sasson S, Stark AH, Bernath K, Seger R, Madar Z. Hyperglycemia reduces nitric oxide synthase and glycogen synthase activity in endothelial cells. Nitric Oxide. 2002;7:187–193. doi: 10.1016/S1089-8603(02)00106-4. [DOI] [PubMed] [Google Scholar]
- Sambuy Y, Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- Schedl HP, Christensen KK, Ronnenberg WC. Effects of diabetes on calcium uptake by rat brush border membrane vesicles. Clin Exp Pharmacol Physiol. 1995;22:272–276. doi: 10.1111/j.1440-1681.1995.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Physiol. 1999;277:E760–E771. doi: 10.1152/ajpendo.1999.277.4.E760. [DOI] [PubMed] [Google Scholar]
- Thomson AB, Wild G. Adaptation of intestinal nutrient transport in health and disease. Part II. Dig Dis Sci. 1997;42:470–488. doi: 10.1023/A:1018874404762. [DOI] [PubMed] [Google Scholar]
- Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharm Res. 1997;14:763–766. doi: 10.1023/A:1012102522787. [DOI] [PubMed] [Google Scholar]