Abstract
The SurePath liquid-based Pap test (LPT) is successfully and widely used to assess sputum cytology. This study aimed to compare the cytological findings and diagnostic sensitivity of LPT with those of the conventional Pap smear (CPS) method for diagnosing lung cancer. Bronchial brushing specimens from 204 patients diagnosed with lung cancer were studied. LPT slides showed decreased areas of cell monolayers, a clearer background and distinct, stereoscopic cytological features. The LPT had a significantly higher diagnostic sensitivity for lung cancer (71.6%) than the CPS method (57.8%, p < 0.05), particularly for small cell lung carcinoma and >2 cm lesions (p < 0.05). Combination of the LPT with the CPS method showed obviously higher diagnostic sensitivity for the detection of adenocarcinoma (63.6%), central lesions (85.0%) and >2 cm lesions (81.4%) compared with the CPS method alone (p < 0.05, p < 0.01). Thus, LPT is a useful and easily performed technique that can be widely applied, and is suitable for the early diagnosis of lung cancer.
Keywords: Cytopathology, Liquid-based Pap test, Lung cancer, Bronchial brushing, Cytodiagnosis
To establish a cytologic or histologic diagnosis in a patient with suspected lung cancer, flexible bronchoscopy is an essential step in the workup (Van et al. 2005). Brushings, washings and forceps biopsies are often combined to increase the diagnostic yield (Saltzstein et al. 1977; Mak et al. 1990). Brushing complements forceps biopsy alone in the diagnostic workup of lung cancer and can be useful in the identification of lung carcinoma, a combination of brushing and forceps biopsy is the best strategy in the diagnosis of bronchoscopically visible lung cancer (Matsuda et al. 1986; Karahalli et al. 2001). However, the literature differs regarding both the sensitivities of the diagnoses and the conclusions for the utility value of flexible bronchoscopy (Karahalli et al. 2001; Lam et al. 2000; Roth et al. 2008), the major limitation of the preparation method has been variable quality due to contamination with blood, cellular debris and mucus hiding the tumor cells (Motherby et al. 1999). The liquid-based cytological test (LPT) is a useful cytological technique and its clinical application was approved by Food and Drug Administration in 1999. It is successfully used in sputum cytology for the preparation of clear cell monolayers through the removal of mucus, necrotic material and inflammatory cells (Wu et al. 2009; Choi et al. 2008).
This study aimed to directly compare the LPT with the conventional pick-and-smear (CPS) method, with respect to the quality of the slides and diagnostic sensitivity. The use of the LPT for the diagnosis of lung cancer was also evaluated.
Methods
The present study was conducted in accordance with the regulations of the institutional review boards at China Medical University and performed between January and October 2008 at The First Affiliated Hospital, China Medical University. Two hundred and four patients were diagnosed with lung cancer based on histopathology of bronchial biopsy and surgical resection of the tumors. These 204 patients with lung cancer, as well as 40 randomly selected patients without lung cancer (28 with endobronchial tuberculosis and 8 with hyperplasia and 4 with inflammatory pseudotumor), were included as study subjects and control subjects, respectively. In the study group there were 120 men and 84 women, ranging in age from 31 to 79 years. Histological examination confirmed that there were 104 cases of squamous cell carcinoma (SCC), 44 cases of adenocarcinoma (AC), and 56 cases of small cell lung carcinoma (SCLC).
All bronchoscopies were performed by two experienced bronchoscopists. The most common reasons for bronchoscopy were an abnormal chest radiograph, endobronchial lesions were classified as mass, submucosal lesion and infiltration. Forceps biopsies and brushings were obtained from all subjects, included 3–4 forceps biopsies and 4 endobronchial brushings, two direct smears were prepared from brushing and immediately fixed in 95% ethanol and stained with Papanicolaou. Residual brushing was transferred to a vial with SurePathTM preservative fluid (BD Tripath, Burlington NC, USA). One millilitre of the mucolytic agent (BD Tripath) was added to the vial with SurePathTM preservative fluid, incubated at room temperature for 30 min and vortexed for 10 s. Additional mucolytic agent was added to the mixture until the mucus was completely lysed.
The mixed liquid was then transferred to a 50-ml tube and centrifuged at 2,000g for 10 min. The supernatant was removed and the pellet was resuspended in 7.5 ml of distilled water. This suspension was vortexed again and centrifuged at 2,000g for 5 min. The supernatant was removed and the pellet was vortexed and transferred to the AutoCyte PREP system (BD Tripath), in which slides were automatically prepared and stained. Two slides were prepared from each tube and were stained with Papanicolaou.
For all brushing specimens, slides prepared by both the LPT and conventional CPS methods were screened and assessed independently by two cytopathologists, if the results of diagnosis were different, then they had to review the slides till they reached an agreement.
The sensitivities of the two methods were compared by the χ2 test using the SPSS 11.5 software package. Statistical significance was defined as p < 0.05.
Results
In comparison with the CPS method, LPT method demonstrated a number of advantages. First, screening time was reduced and screening efficiency was increased due to the decreased areas of cell monolayers. Second, the slides had a clearer background due to the dissolution of mucous material, the destruction of most of the red blood cells and significantly reduced numbers of inflammatory cells. Thus, abnormal and tumor cells were more readily discernible. Regardless of the cell type, the cells were clearly stained and their morphology was well preserved. The microscopic fine structure of the nuclear envelope, nucleoli and chromatin were also clearly discernible and stereoscopic.
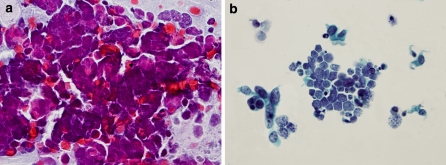
In the LPT slides, SCC cells were evenly distributed in a perfect monolayer pattern, without mucus (Fig. 1); Single AC cells showed globular and stereoscopic morphology in the LPT slides and small aggregates of AC cells were arranged in an acinic pattern (Fig. 2); SCLC cells showed mulberry structures (Fig. 3). A comparison of slide quality between the CPS method and the LPT is shown in Table 1.
Fig. 1.
Squamous cell carcinoma. a A slide prepared by the CPS method; b A slide from the same patient prepared with the LPT, showing homogeneous cell distribution in a perfect monolayer and absence of mucus (Papanicolaou stain, × 400)
Fig. 2.
Adenocarcinoma. a A slide prepared by the CPS method; b A slide from the same patient prepared with the LPT, showing a clearer background. The small aggregates of cancer cells are arranged in an acinic pattern (Papanicolaou stain, × 400)
Fig. 3.
Small cell lung carcinoma. a A slide prepared by the CPS method; b A slide from the same patient prepared with the LPT, showing a clearer background, crowded cell clusters, small uniform nuclei and scanty cytoplasm (Papanicolaou stain, × 400)
Table 1.
Comparison of the difference in quality between slides generated by the CPS method and the LPT for the identification of cancer cells
| Methods | CPS | LPT |
|---|---|---|
| SCC | Large amounts of mucus, necrotic material, inflammatory cells and red blood cells were observed among the SCC cells | The original cellular structures were well preserved, without mucus or white blood cells among the SCC cells |
| AC | Mucus and inflammatory cells were observed around AC cells, which showed silkworm-like, spindle structures | The small aggregates of AC cells were arranged in an adenoid pattern and had stereoscopic morphology |
| SCLC | The dispersed cells were arranged in strip or band structures | The crowded cell clusters showed mulberry or inlay-like structures |
SCC squamous cell carcinoma; AC adenocarcinoma; SCLC small cell lung carcinoma
Table 2 shows comparisons of the LPT, CPS and combination of the LPT and CPS methods for detecting lung cancer. The results showed that combination of the LPT and CPS methods increased the sensitivity for all lung cancer and most of the gain in sensitivity was due to the LPT. There were significant gains for the detection of adnocarcinoma and small cell carcinoma but not for squamous cell carcinoma. Of the 204 brushing samples from patients with lung cancer examined by the conventional CPS method, only 118 were found to have cancer cells, duo to obscuring by mucous material, inflammatory cells and necrotic debris. The diagnostic sensitivity was only 57.8%. However, the LPT showed 71.6% diagnostic sensitivity duo to a clearer background and well preserved cell morphology. This difference in diagnostic sensitivity between the two methods was significant (p < 0.05). When the CPS method and the LPT were evaluated in combination, the diagnostic sensitivity for detecting lung cancer was 76.5%, significantly higher than the CPS method alone (57.8%, p < 0.01). No cancer cells were found in the 40 samples from patients without lung cancer.
Table 2.
Comparison of the LPT, the CPS method, and a combination of LPT and the CPS method for detection of lung cancer by histologic type of lesion
| Pathology | n | LPT | CPS | LPT + CPS | |||
|---|---|---|---|---|---|---|---|
| Positive | Percentage | Positive | Percentage | Positive | Percentage | ||
| SCC | 104 | 80 | 76.9 | 76 | 73.1 | 86 | 82.7 |
| AC | 44 | 24 | 54.5 | 18 | 40.9 | 28 | 63.6* |
| SCLC | 56 | 42 | 75.0* | 24 | 42.9 | 42 | 75.0* |
| Total | 204 | 146 | 71.6* | 118 | 57.8 | 156 | 76.5** |
* p < 0.05, ** p < 0.01, when compared with the CPS method
SCC squamous cell carcinoma; AC adenocarcinoma; SCLC small cell lung carcinoma
Of the 80 positive SCC cases as determined by LPT, ten were negative by the CPS method, and 70 were positive by either the LPT or the CPS method. On the other hand, of the 76 positive SCC cases as determined by the CPS method, six were positive by the CPS method but negative by LPT, and 70 were positive by either LPT or the CPS method. The diagnostic sensitivity for the detection of SCC was 82.7% when the LPT and CPS methods were combined. There was no significant difference when compared with either method alone (p > 0.05).
Of the 24 positive AC cases as determined by LPT, eight were negative by the CPS method and 16 were positive by either LPT or the CPS method. Of the 18 positive AC cases as determined by the CPS method, two were positive by the CPS method but negative by LPT, and 16 were positive by either LPT or the CPS method. Although there was no significant difference in diagnostic sensitivity between the two methods (p > 0.05), when LPT and the CPS method were combined, the diagnostic sensitivity for AC was 63.6%, significantly higher than that of the CPS method alone (p < 0.05).
Of the 42 positive SCLC cases as determined by the LPT method, 24 were positive by the CPS method and the remaining 18 were negative. The diagnostic sensitivity of the LPT for the detection of SCLC was 75.0%, significantly higher than that of the CPS method alone (42.9%, p < 0.05).
Table 3 shows comparisons of the LPT, CPS and combination of the LPT and CPS methods for detecting lung cancer by location and size of lesion. The LPT had a significantly higher diagnostic sensitivity for >2 cm lesions (75.6%) than the CPS method (62.8%); LPT showed obviously higher diagnostic sensitivity for the detection of central lesions (78.6%) than the CPS method (60.3%, p < 0.01).
Table 3.
Comparison of the LPT, the CPS method, and a combination of LPT and the CPS method for detection of lung cancer by location and size of lesion
| Lesion | n | LPT | CPS | LPT + CPS | |||
|---|---|---|---|---|---|---|---|
| Positive | Percentage | Positive | Percentage | Positive | Percentage | ||
| Central | 126 | 99 | 78.6** | 76 | 60.3 | 107 | 85.0** |
| Peripheral | 78 | 47 | 60.3 | 42 | 53.8 | 49 | 62.8 |
| >2 cm | 156 | 118 | 75.6* | 98 | 62.8 | 127 | 81.4** |
| <2 cm | 48 | 28 | 58.3 | 20 | 41.7 | 29 | 60.4 |
| Total | 204 | 146 | 71.6* | 118 | 57.8 | 156 | 76.5** |
* p < 0.05, ** p < 0.01, when compared with the CPS method
Discussion
The role of fiberoptic bronchoscopy in the diagnosis of lung cancer presenting as a bronchoscopically visible lesion has been studied extensively (Arroliga and Matthay 1993). The diagnosis of cancer is usually established by obtaining a forceps biopsy of visible lesions, brushing cytology and bronchial washing (Arroliga and Matthay 1993; Kaparianos et al. 2008). The diagnostic sensitivity of brushing cytology was reported to be quite variable in different institutions, it was reported to be 62–78% (Karahalli et al. 2001; Arroliga and Matthay 1993; Govert et al. 1996) whereas the sensitivity of LPT was 71.6% in the current study. There was significant difference in diagnostic sensitivity between LPT and CPS methods (p < 0.05), and when CPS method and LPT method were combined, the diagnostic sensitivity for the detection of lung cancer was 76.5% and significantly higher than the conventional CPS method (57.8%, p < 0.01).
The well-known Saccomanno method of sputum cytology was used to collect the specimen in a fixative, blend the material to liquefy the mucus and disperse the cells in an evenly distributed monolayer on slides. This method provided more information for the diagnosis of lung cancer and decreased false negative results (Rizzo et al. 1990). However, Perlman et al. (1989) reported that this method was not better than the fresh smear method, particularly for the diagnosis of SCLC. Recently, Tang et al. (1995) used dithiothreitol as a mucolytic agent and found this approach to be more sensitive than the CPS method for the diagnosis of lung cancer. However, this method did not remove inflammatory cells and red blood cells from the slides.
The area of a conventional smear is generally 1,375 mm2 and the time needed for screening is 7 min, whereas the area of the LPT monolayer is only 133 mm2, and the time needed for screening is reduced to 2.5 min. Forty-eight samples could be processed simultaneously and the cells stained automatically using standard procedures in 1 h. The cell monolayer showed a clearer background and stronger contrast qualities due to the programming of specimen processing, slide preparation and staining. Therefore, the cytologists were more easily able to concentrate on screening every field of vision with a notable increase in screening efficiency. This is in agreement with a previous report by Rana et al. (2001).
With the CPS method, SCLC slides contained necrotic material, and showed small cells in loose arrangement. The malignant cells were “flooded” with mucus, epithelial cells and other impurities in the samples, which is hard to be observed, and as a result the diagnostic sensitivity was lower. Wang et al. (2004) reported that the diagnostic sensitivity for SCLC was 50% when using the sputum processing method. However, the diagnostic sensitivity of SCLC by LPT method was significantly higher in our previous study (Wu et al. 2009), the present study showed that with the LPT the diagnostic sensitivity for SCLC increased to 75.0% from 42.9% observed with the CPS method. The LPT therefore represent a useful method of bronchial secretions processing not previously used in diagnostic cytology, and could be a potentially powerful tool for the diagnosis of SCLC.
In this study, the epithelial cells were deposited naturally on glass slides coated with poly-l-lysine in an automatic procedure. There was no smearing or distortion, the cells were distinctly stereoscopic and cellular structures were well preserved, especially in the AC slides. Eighteen specimens were positive for AC when examined using the CPS method, whereas 24 specimens were positive with the LPT. Although there was no significant difference in diagnostic sensitivity between the two methods, the diagnostic sensitivity for AC was 63.6% when the CPS method and LPT were combined, which is significantly higher than that for the CPS method alone (40.9%, p < 0.05).
On the other hand, the diagnostic sensitivity of the LPT for SCC was not as good. The number of cases positively diagnosed using the CPS method was 76, whereas with the LPT only 80 cases were positively diagnosed. This may have been due to the large number of squamous cells obtained with the CPS method (Choi et al. 2008), and the fact that the number of monolayer slides prepared for each specimen may not have been sufficient. In the cases of the eight SCC and one AC specimen examined using the LPT, the first slide showed no cancer cells, although carcinoma cells were found on the second one. This is likely to be due to the non-globular morphology of SCC cells, which are not readily sedimented and therefore lost during centrifugation. This is in agreement with our previous report for the test of sputum cytology (Wu et al. 2009).
On comparisons of the LPT, CPS and combination of the LPT and CPS methods for detecting lung cancer by location and size of lesion, the LPT had a significantly higher diagnostic sensitivity for >2 cm lesions (75.6%) than the CPS method (62.8%, p < 0.05); LPT showed obviously higher diagnostic sensitivity for the detection of central lesions (78.6%) than the CPS method (60.3%, p < 0.01). This is in agreement with a previous report by Schreiber and McCrory (2003).
Motherby et al. (1999) reported a diagnostic sensitivity of 82.6% for the diagnosis of lung tumors if one slide was prepared from the bronchial secretions, 88.8% with two slides and 94.0% with seven to eight slides. This indicates that a single slide may not adequately represent all the cellular elements from a brushing specimen. We suggest that at least four slides be prepared for each sample. Further studies are needed to use all residual material and investigate the diagnostic accuracy of LPT in detecting cancer cells in bronchial secretions from patients with lung cancer.
The LPT provides automatic preparation of cell monolayer from bronchial brushing that are highly representative of the cellular content of brushing samples and are superior to those obtained by the CPS method. LPT results in a clearer background, smaller areas to be screened and well preserved, distinctly stereoscopic original cellular structures. The method is therefore suitable for the early diagnosis of lung cancer.
Acknowledgments
This work was supported by grant from the Liaoning Science and Technology Project (Wu Guang-Ping, Grant No.2008225021-3).
Contributor Information
Yi-Bo Fan, Phone: +86-24-83282248, FAX: +86-24-83282248, Email: fany_bo@hotmail.com.
Qing-Shan Wang, Phone: +86-24-23901480, FAX: +86-24-23901480, Email: wqssj@yahoo.com.cn.
Lin Ye, Phone: +86-24-83282248, FAX: +86-24-83282248, Email: yel_gy@sina.com.
Tian-Yu Wang, Phone: +86-24-83282248, FAX: +86-24-83282248, Email: wangt_yu@sina.com.
Guang-Ping Wu, Phone: +86-24-83282248, FAX: +86-24-83282248, Email: wug_ping@sina.com.
References
- Arroliga AC, Matthay RA. The role of bronchoscopy in lung cancer. Clin Chest Med. 1993;14:87–98. [PubMed] [Google Scholar]
- Choi YD, Han CW, Kim JH, Oh IJ, Lee JS, Nam JH, Juhng SW, Park CS. Effectiveness of sputum cytology using ThinPrep method for evaluation of lung cancer. Diagn Cytopathol. 2008;36:167–171. doi: 10.1002/dc.20761. [DOI] [PubMed] [Google Scholar]
- Govert JA, Kopita JM, Matchar D, Kussin PS, Samuelson WM. Cost-effectiveness of collecting routine cytologic specimens during fiberoptic bronchoscopy for endoscopically visible lung tumor. Chest. 1996;109:451–456. doi: 10.1378/chest.109.2.451. [DOI] [PubMed] [Google Scholar]
- Kaparianos A, Argyropoulou E, Sampsonas F, Zania A, Efremidis G, Tsiamita M, Spiropoulos K. Indications, results and complications of flexible fiberoptic bronchoscopy: a 5-year experience in a referral population in Greece. Eur Rev Med Pharmacol Sci. 2008;12:263–355. [PubMed] [Google Scholar]
- Karahalli E, Yilmaz A, Türker H, Ozvaran K. Usefulness of various diagnostic techniques during fiberoptic bronchoscopy for endoscopically visible lung cancer: should cytologic examinations be performed routinely? Respiration. 2001;68:611–614. doi: 10.1159/000050581. [DOI] [PubMed] [Google Scholar]
- Lam B, Wong MP, Ooi C, Lam WK, Chan KN, Ho JC, Tsang KW. Diagnostic yield of bronchoscopic sampling methods in bronchial carcinoma. Respirology. 2000;5:265–270. doi: 10.1046/j.1440-1843.2000.00258.x. [DOI] [PubMed] [Google Scholar]
- Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fibreoptic bronchoscopy in the diagnosis of lung cancer. Thorax. 1990;45:373–376. doi: 10.1136/thx.45.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Horai T, Nakamura S, Nishio H, Sakuma T, Ikegami H, Tateishi R. Bronchial brushing and bronchial biopsy: comparison of diagnostic accuracy and cell typing reliability in lung cancer. Thorax. 1986;41:475–478. doi: 10.1136/thx.41.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motherby H, Nicklaus S, Berg A, Ohler S, Ross B, Sarbia M, Böcking A. Semiautomated monolayer preparation of bronchial secretions using AutoCyte PREP. Acta Cytol. 1999;43:47–57. doi: 10.1159/000330868. [DOI] [PubMed] [Google Scholar]
- Perlman EJ, Erozan YS, Howdon A. The role of the Saccomanno technique in sputum cytopathologic diagnosis of lung cancer. Am J Clin Pathol. 1989;91:57–60. doi: 10.1093/ajcp/91.1.57. [DOI] [PubMed] [Google Scholar]
- Rana DN, O’Donnell M, Malkin A, Griffin M. A comparative study: conventional preparation and ThinPrep 2000 in respiratory cytology. Cytopathology. 2001;12:390–398. doi: 10.1046/j.1365-2303.2001.00351.x. [DOI] [PubMed] [Google Scholar]
- Rizzo T, Schumann GB, Riding JM. Comparison of the pick-and-smear and Saccomanno methods for sputum cytologic analysis. Acta Cytol. 1990;34:875–880. [PubMed] [Google Scholar]
- Roth K, Hardie JA, Andreassen AH, Leh F, Eagan TM. Predictors of diagnostic yield in bronchoscopy: a retrospective cohort study comparing different combinations of sampling techniques. BMC Pulm Med. 2008;8:2. doi: 10.1186/1471-2466-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzstein SL, Harrell JH, Cameron T. Brusings, washings, or biopsy? Obtaining maximum value from flexible fiberoptic bronchoscopy in the diagnosis of cancer. Chest. 1977;71:630–632. doi: 10.1378/chest.71.5.630. [DOI] [PubMed] [Google Scholar]
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123:115S–128S. doi: 10.1378/chest.123.1_suppl.115S. [DOI] [PubMed] [Google Scholar]
- Tang CS, Tang CM, Lau YY, Kung IT. Sensitivity of sputum cytology after homogenization with dithiothreitol in lung cancer detection. Two years of experience. Acta Cytol. 1995;39:1137–1140. [PubMed] [Google Scholar]
- Drift MA, Wilt GJ, Thunnissen FB, Janssen JP. A prospective study of the timing and cost-effectiveness of bronchial washing during bronchoscopy for pulmonary malignant tumors. Chest. 2005;128:394–400. doi: 10.1378/chest.128.1.394. [DOI] [PubMed] [Google Scholar]
- Wang GS, Lee YC, Perng RP. A novel method of sputum processing for cytologic diagnosis of lung cancer. Anal Quant Cytol Histol. 2004;26:121–126. [PubMed] [Google Scholar]
- Wu GP, Wang EH, Li JH, Fu ZM, Han S. Clinical application of the liquid-based cytological test in cytological screening of sputum for the diagnosis of lung cancer. Respirology. 2009;14:124–128. doi: 10.1111/j.1440-1843.2008.01399.x. [DOI] [PubMed] [Google Scholar]