Abstract
Purpose
The aim of this study is to assess the incidence and natural history of cavitating lung lesions in chronic thromboembolic pulmonary hypertension (CTEPH), note thrombus position between patients with and without a cavity and determine whether their development is a predictor of mortality.
Materials & Methods
All patients with confirmed CTEPH attending our Pulmonary Vascular Unit between February 1998 and January 2006 were identified, and a review of their notes and imaging was performed. Thrombus position, pre-disposing factors, cavity progression and mortality were noted, and comparisons made between those with and without a cavity.
Results
11 of 104 patients had a cavity (10.6%). Thrombus distribution was similar between those with and those without a cavity. Preceding infection was not proven in most cases. 27.3% of patients with a cavity died compared to 26.8% of those without.
Conclusion
Cavity formation in CTEPH is 3 times more common than in acute pulmonary embolism. Thrombus position does not predict cavity development, and the presence of a cavity may serve as an indicator of disease severity but does not appear to predict mortality.
INTRODUCTION
Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure (PAP) of >25 mmHg at rest, with an elevation of peak systolic pressure to >30 mmHg during exercise (1,2,3). Fibromuscular hypertrophy of the intima, media and adventitia occurs which narrows and finally obliterates the vessel lumen (1,4), reducing the cross-sectional area of the pulmonary vascular bed and increasing pulmonary vascular resistance. At presentation, up to 80% of pulmonary vessels have been obliterated, and survival from diagnosis is poor (1,5,6). Pulmonary hypertension has many causes and classification has recently been updated (Table 1) (7). Diagnosis and treatment is complex, and all patients with suspected PH should be referred to a tertiary centre. PAP is initially estimated by echocardiography, and confirmed by a diagnostic right heart catheter (8,9). Radiological investigations include plain chest radiograph (CXR), lung scintigraphy, computed tomography pulmonary angiogram (CTPA) and high resolution computed tomography (HRCT) of the thorax. Conventional pulmonary angiography is occasionally performed (3,10). CXR is often normal in PH, but abnormal findings include enlarged pulmonary arteries and pruning of peripheral vessels. Lung scintigraphy demonstrates perfusion defects, while CTPA and HRCT assess pulmonary arterial obstruction and lung parenchyma for underlying disease or changes secondary to pulmonary hypertension e.g. infarction, consolidation, mosaic perfusion patterns. Magnetic resonance imaging (MRI) aimed at quantifying pulmonary artery pressure in a non-invasive way is also being developed, but at present is mainly a research tool (8,9,11). Treatment depends on disease severity. All patients receive anticoagulation (6), and vasodilators are also usually prescribed (1,6,12,13). Surgical thromboendarterectomy is considered for chronic thromboembolic pulmonary hypertension, while heart-lung transplant is reserved for severe cases.
Table 1.
Revised Evian Nomenclature and Classification of Pulmonary Hypertension (Venice Symposium 2003)
| Pulmonary arterial hypertension | Idiopathic | |
| Familial | ||
| Secondary to | Collagen vascular disease | |
| Congenital systemic to pulmonary shunts | ||
| Portal hypertension | ||
| HIV infection | ||
| Drugs/toxins | ||
| Other | ||
| Pulmonary veno-occlusive disease | ||
| Pulmonary capillary haemangiomatosis | ||
| Pulmonary venous hypertension | Left-sided atrial or ventricular heart disease | |
| Left-sided valvular heart disease | ||
| PH associated with hypoxemia | COPD | |
| Interstitial lung disease | ||
| Sleep-disordered breathing | ||
| Alveolar hypoventilatory disorders | ||
| Chronic exposure to high altitude | ||
| PH due to chronic thrombotic and/or embolic disease | Thromboembolic obstruction of proximal pulmonary arteries | |
| Obstruction of distal pulmonary arteries | ||
| Pulmonary embolism | thrombus, tumor, foreign material | |
| Pulmonary capillary hemangiomatosis | ||
| Other | ||
| Miscellaneous | Extrinsic compression of central pulmonary veins | Fibrosing mediastinitis |
| Adenopathy/tumors | ||
| Sarcoidosis | ||
| Histiocytosis X | ||
| Lymphangiomatosis |
Right heart failure is the commonest complication and cause of death in these patients, but other recognized sequelae include central arterial atherosclerosis and thrombosis, aneurysmal pulmonary artery dilatation and lung infarction (3,5,14,15). Amongst some of our patients with chronic thromboembolic pulmonary hypertension (CTEPH), we have also observed formation of pulmonary cavities. This is a well-recognized complication in acute thromboembolic disease (16,17,18,19,20,21), but cavity formation has not been previously documented in CTEPH.
The aim of this study is to assess how often we encounter cavitating lung lesions in patients with CTEPH, note thrombus position in patients with and without a cavity, note any associated infective organisms, demonstrate the findings and course of these lesions and determine whether their development is an indicator of poor outcome. Comparison with cavity characteristics in acute thromboembolic disease is also made.
MATERIALS AND METHODS
Between February 1998 and January 2006, 104 patients attended our tertiary referral unit for further investigation and management of CTEPH. All patients underwent echocardiography, ISWT, right heart catheterization, CXR and CTPA combined with HRCT. Some also had conventional pulmonary angiography and MRI. All patients who developed lung cavities were identified and a review of the notes (RB/DK) and imaging (HH/EVB) was performed. All referred patients were followed up. Thrombus position (proximal or distal) and mortality data for those with and without cavities was obtained. Of the patients who developed a cavity, symptoms at time of cavity diagnosis were noted along with inflammatory markers and sputum culture. Cavity characteristics were documented along with the radiological course in those which were followed up.
RESULTS/CASES
A total of 11 of the 104 patients (10.6%) developed lung cavities. Mortality rates between those that developed cavities (three died; 27.3%) was comparable to those that did not develop cavities (25 of 93 died; 26.8%) The thrombus distribution between these two groups was also similar, with distal thrombus present in 22/93 (23.7%) patients without cavity compared to 3/11 (27.3%) patients with cavitary lesions. A summary of radiological features in shown in tables 2 & 3.
Table 2.
Radiological features associated with the cavities
| Patient | Underlying pathology | Imaging Modality of 1st cavity diagnosis | Number of Cavities | Cavity Size and Location | Other Cavity Features | Infective Organism in Sputum | Additional CTPA findings |
|---|---|---|---|---|---|---|---|
| Case 1 | CTEPH | CTPA | 2 | 4cm Left upper lobe |
Cavity within area of consolidation | Pseudomonas aeroginosa Enterococcus | Extensive proximal pulmonary artery thrombus |
| 3cm Right posterior basal segment |
Cavity within area of consolidation, containing air/fluid level. | ||||||
| Case 2 | CTEPH | CT Thorax | 1 | 3cm Right lower lobe |
Cavity within an area of soft tissue opacification | MRSA | Right pulmonary artery mural thrombus |
| Case 3 | CTEPH | Chest X-Ray and CT thorax | 1 | 5cm Right lower lobe apical segment |
Thick-walled cavity surrounded by consolidation | Pseudomonas aeroginosa | Right pulmonary artery thrombus |
| Case 4 | CTEPH | CT thorax | 1 | Small Right upper lobe |
Cavity within area of consolidation | No | Right lower lobe pulmonary artery thrombus |
| Case 5 | ASD with Eisenmengers CTPA and CTEPH | Chest X-Ray and | 1 | 6cm Right upper lobe |
Cavity within an area of consolidation. | No | Thrombus in right main and upper lobe arteries |
| Pruning of right upper lobe pulmonary artery | |||||||
| Case 6 | CTEPH | CTPA | 1 | Small Apical segment left lower lobe |
Cavity within area of consolidation | No | Thrombus in inferior lingula and right middle and lower lobe arteries |
| Case 7 | CTEPH | Chest X-Ray and CTPA | 1 | 2.2cm Right lower lobe apical segment |
Thick-walled cavity within consolidation | No | Enlarged main pulmonary artery |
| Case 8 | CTEPH | CTPA | 2 | 4.4cm & 3.3cm Right upper lobe anterior segment |
Both cavities thin-walled consolidation | No | Multiple pulmonary emboli in arteries leading to area of consolidation |
| Enlarged main pulmonary artery | |||||||
| Case 9 | CTEPH | CTPA | 1 | 0.8cm Right apex |
Thin-walled cavity No consolidation |
No | Mosaic pattern |
| Enlarged pulmonary artery with proximal thrombus | |||||||
| Case 10 | CTEPH | CTPA | 1 | 4cm Right base lower lobe |
Thick-walled cavity No consolidation |
No | Mosaic pattern. Pulmonary artery diameter 4.7cm. |
| Case 11 | CTEPH | CTPA | 3 | Moderate Right posterior apex |
Irregular, thick-walled cavity | No | Extensive thrombus in main pulmonary artery and right lower lobe artery. Marked pruning of both lower lobe segmental arteries. Pulmonary artery diameter 3.6cm |
| 2 × small cavites Both lower lobes |
Irregular, thick-walled cavities | ||||||
Table 3.
Table demonstrating the natural progression of the followed-up cavities
| Patient | Size | Wall Thickness | Cavity Fluid | New Cavities |
|---|---|---|---|---|
| Case 1 | 4cm cavity ↑ | ↓ | no | Yes Right upper lobe |
| 3cm cavity ↑ with partial collapse | ↓ | Resolved | ||
| Case 2 | ↑ to 8×5cm | ↑ | Yes | no |
| Then gradual ↓ and collapse | gradual ↓ | resolved | ||
| Case 3 | ↓ then resolved | ↓ | no | no |
| Case 4 | Resolved at follow-up scan | ↓ | no | no |
| Case 5 | Small ↓ | No change | no | no |
| Then cavity size remained static | No change | |||
| Case 7 | Resolved at follow-up scan | ↓ | no | no |
| Case 8 | Both cavities ↓ in size, and then resolved | ↓ | no | no |
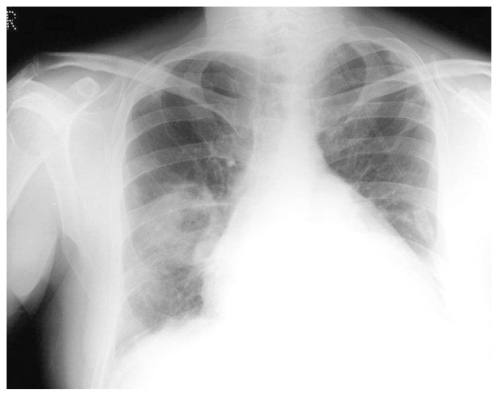
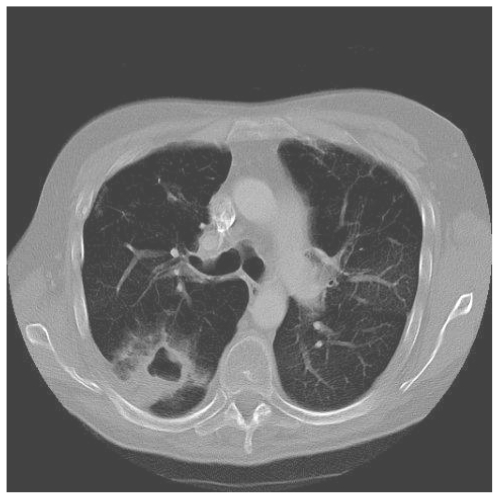
Case 1
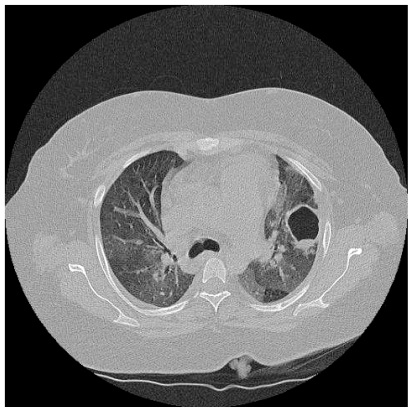
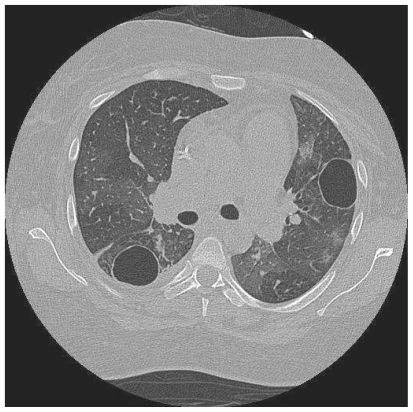
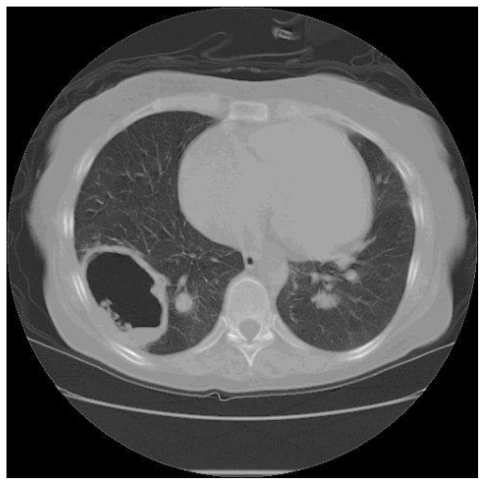
A 35 year old woman presented with two years of progressive exertional dyspnoea. Right heart catheterisation showed significant PH with a mean PAP of 41 mmHg, and CTPA demonstrated extensive proximal thrombus. Anticoagulation and vasodilators were commenced, but a high BMI and poor lung function made her unsuitable for pulmonary endarterectomy. Sixteen months later, she was readmitted with increasing peripheral oedema and haemoptysis. Two lung cavities were seen on CT thorax lying within areas of consolidation (Fig 1a). 1 was in the left upper lobe measuring 4cm in diameter, while the other measured 3 cm within the posterior basal segment of the right lower lobe, containing an air-fluid level. C-Reactive Protein (CRP) was elevated and sputum culture grew Pseudomonas and an Enterococcus. Clinical improvement followed antibiotic treatment. CT four months later showed increased size in both cavities, with thinner walls and partial collapse of the right basal cavity with resolution of intra-cavitary fluid (Fig 1b). A third cavity was seen in the right upper lobe. She died while awaiting transplant assessment.
Figure 1a.
HRCT demonstrating a 4cm thick walled cavity in the left upper lobe surrounded by patchy consolidation and containing fluid. Notice mosaic perfusion present in the lower lobe.
Figure 1b.
HRCT performed 5 months later shows that both cavities have increased in size, with thinning of the cavity wall and resolution of the intracavitary fluid.
Case 2
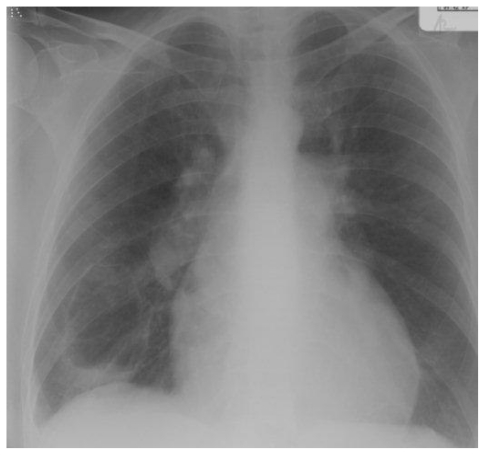
A 57 year old woman presented with progressive exertional dyspnoea, chest tightness and intermittent haemoptysis. CTPA showed mural thrombus in the right main pulmonary artery and right heart catheterisation confirmed severe CTEPH, with a mean PAP of 72 mmHg. Anticoagulation and vasodilators were commenced. Four months later a 3cm cavity was seen on CT at the right lung base, lying within an area of consolidation (fig. 2a). CRP was elevated and MRSA was cultured from sputum. She responded to antibiotics. MRI and MR pulmonary angiography (MRPA) were performed, demonstrating biventricular hypertrophy, massive right atrial enlargement, enlarged pulmonary trunk with slow flow and mural thrombus, and extensive pulmonary arterial stenoses and post-stenotic webs, consistent with CTEPH (fig. 2b–c). The cavity was also seen. Five months later, CT showed the cavity had increased in size to 8×5cm, was now thick walled and contained a small amount of fluid (fig. 2d). Serial CT and CXR follow-up of the cavity demonstrated a steady decrease in cavity size and wall thickness (fig. 2e–f), with eventual cavity collapse. The patient died from complications of her disease.
Figure 2a.
HRCT showing a 3cm cavity within an area of soft tissue in the base of right lower lobe
Figure 2b.
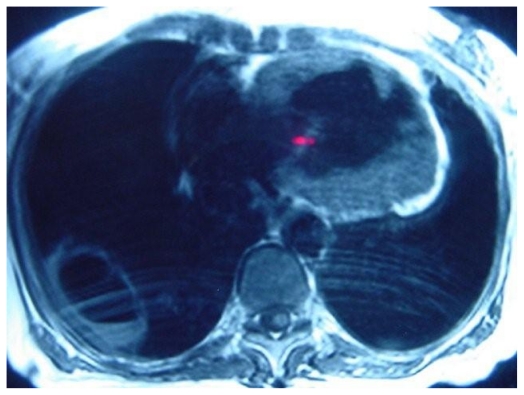
MR pulmonary angiogram in this patient with severe pulmonary hypertension, demonstrating enlarged main pulmonary trunk, multiple arterial stenoses and post-stenotic webs, which are diagnostic of chronic thromboembolic pulmonary hypertension
Figure 2c.
Proton MR demonstrating right ventricular hypertrophy and cavity with air-fluid level.
Figure 2d.
CT thorax 5 months later shows the cavity has enlarged to 8×5cm, is thick-walled and contains a small amount of fluid
Fig 2e.
Chest radiograph demonstrating the same cavity.
Figure 2f.
Radiographic follow-up performed 16 months later showing continuing cavity involution
Case 3
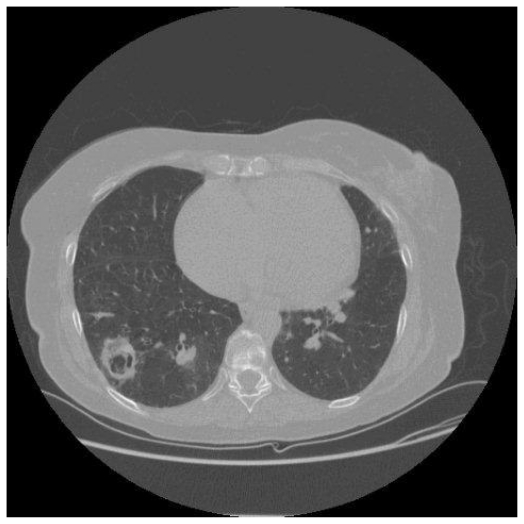
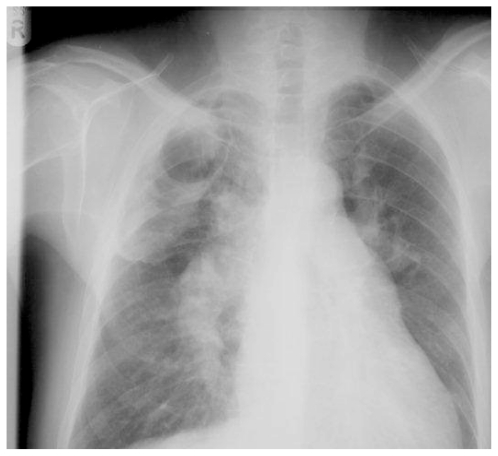
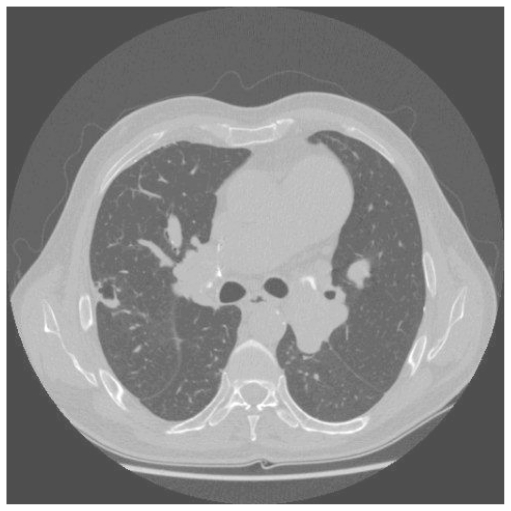
A 49 year old man developed exertional dyspnoea. A pulmonary embolus was diagnosed and he was anticoagulated. His dyspnoea did not improve, and right heart catheterisation showed significant PH with a mean PAP of 44 mmHg. CTPA demonstrated thrombus in the right central pulmonary arteries. Anticoagulation and vasodilators were commenced. He was readmitted, four months later, with cough, haemoptysis and worsening dyspnoea. A large cavity was seen in the right midzone on CXR (fig. 3a). CTPA confirmed this to be thick-walled, lying within an area of consolidation in the apical segment of the right lower lobe (Fig. 3b). CRP was elevated with pseudomonas on sputum culture. Antibiotics produced clinical improvement with a decrease in cavity size within two weeks and complete resolution after four weeks. Subsequently, he underwent successful pulmonary endarterectomy.
Figure 3a.
Chest radiograph showing a large cavity in the right midzone lying within an area of consolidation
Figure 3b.
A corresponding CT thorax demonstrates a 5cm cavity lying within an area of consolidation in the apical segment of the right lower lobe
Case 4
A 66 year old man was referred with progressive dyspnoea following a previous pulmonary embolus. Right heart catheterisation showed a mean PAP of 53 mmHg. CTPA and conventional pulmonary angiography demonstrated widespread segmental embolic disease. MRI showed acute tapering of vessels, vascular cut-off within the right middle lobe and lingula, and slow flow within the main pulmonary trunk. After fifteen months, he presented acutely with increasing dyspnoea and a productive cough. He was afebrile, but CRP was elevated and CXR revealed bilateral upper zone consolidation. Sputum culture was negative. CT thorax confirmed consolidation in the superior segment of the left lower lobe, and the right upper lobe where there was a small cavity. Antibiotics were prescribed, and repeat CT two months later showed resolution of the cavity with replacement by a linear scar. The patient died from disease complications.
Case 5
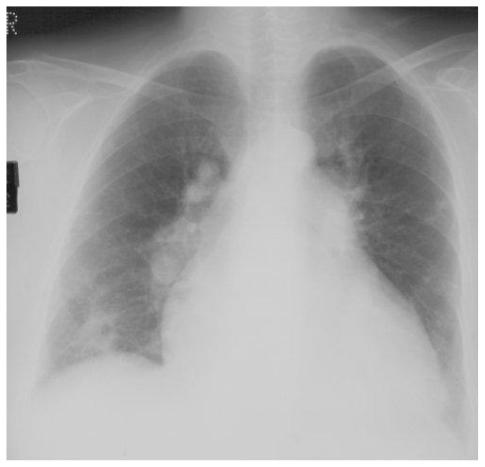
A 55 year old man with an atrial septal defect and associated Eisenmenger syndrome developed progressive exertional dyspnoea with intermittent haemoptysis. Echocardiography was suggestive of significant pulmonary hypertension, but right heart catheterisation was not performed. One year later, he was admitted with worsening dyspnoea and right-sided chest pain. Right upper lobe consolidation containing a 6 cm cavity was seen on CXR, along with marked pruning of the right upper lobe pulmonary artery (fig. 4a). CTPA demonstrated a cavity with well-defined walls and extensive thrombus in the right main and upper pulmonary arteries (Fig. 4b). CTEPH was diagnosed. CRP was elevated and sputum cultures were negative. Antibiotics and warfarin produced a good clinical response. A repeat CXR five weeks later showed a small decrease in size of both the area of consolidation and the cavity but six months on, there was little further change. He is currently on the waiting list for transplantation.
Figure 4a.
Chest radiograph demonstrates a 6cm cavity in the right upper lobe, lying within an area of consolidation, and associated pruning of the upper lobe pulmonary arteries in a generally enlarged pulmonary arterial tree with cardiomegaly.
Figure 4b.
CTPA showing dilated pulmonary arteries, with extensive calcified soft tissue within the right pulmonary artery representing healing thrombus
Case 6
A 43 year old man developed a swollen leg and exertional dyspnoea. Deep venous thrombosis was confirmed by Duplex ultrasonography and lung scintigraphy demonstrated multiple bilateral defects. Lifelong anticoagulation was commenced, but he remained breathless on exertion. Right heart catheterisation demonstrated a mean PAP of 34 mmHg with a dramatic increase on exercise to 68mmHg. CTPA performed on the same admission for clinical work-up showed thrombus within the inferior lingular, right middle lobe and right lower lobe medial basal segmental pulmonary arteries, and a cavity in the apical segment of the left upper lobe. CRP was normal. The patient is awaiting pulmonary endarterectomy.
Case 7
A 69 year old man presented with exertional dyspnoea and an echocardiogram suggestive of pulmonary hypertension. Warfarin was commenced and right heart catheterisation confirmed significant PH with a mean PAP of 49 mmHg. Conventional pulmonary angiogram demonstrated surgically inaccessible chronic thromboembolic disease. Vasodilators were commenced. 16 months after initial diagnosis, he developed a productive cough, and a CXR showed a cavity within right upper lobe consolidation. He was treated with antibiotics and subsequent CT thorax showed cavity resolution with minor residual scarring.
Case 8
A 43 year old lady presented with exertional dyspnoea. 3 months later, she was admitted with worsening breathlessness, cough and pleuritic chest pain. CXR showed right mid zone consolidation and an echocardiogram suggested pulmonary hypertension. CTPA demonstrated pulmonary emboli in the arteries leading to two cavities in the right mid zone, and some lung fibrosis. An initial diagnosis of pulmonary embolism was made and right heart catheterisation confirmed PH with a mean PAP of 53 mmHg. Warfarin and antibiotics were prescribed. Follow-up CT thorax 18 months later demonstrated cavity resolution, with an increase in fibrotic changes.
Case 9
A 70 year old lady with a previous history of pulmonary embolism was admitted with a short history of exertional dyspnoea. An echocardiogram suggested pulmonary hypertension. This was confirmed on right heart catheterisation with a mean PAP of 67 mmHg. A CTPA on the same admission showed proximal thromboembolic disease, while HRCT revealed mosaic perfusion and a small cavity at the right apex. Anticoagulation and vasodilators were commenced, and she is currently awaiting pulmonary endarterectomy.
Case 10
A 51 year old man presented with increasing exertional dyspnoea. CTPA demonstrated distal pulmonary arterial thrombus and a 4cm thick-walled cavity in the base of the right lower lobe, and right heart catheterisation confirmed PH with a mean PAP of 58mmHg. CRP and sputum culture were negative. Antibiotics and vasodilators were commenced and the patient is currently under follow-up.
Case 11
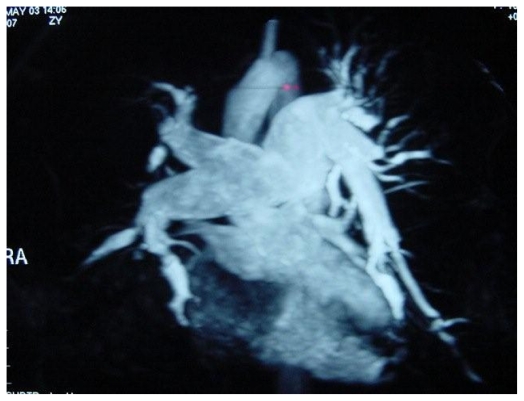
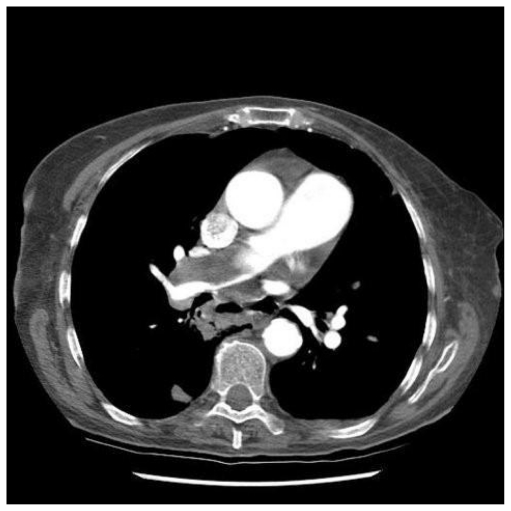
A 77 year old lady presented with progressive breathlessness. Following an acute deterioration, CTPA showed extensive proximal pulmonary arterial thromboembolic disease (fig. 5). Initial improvement followed anticoagulation, but then her symptoms deteriorated, with increasing breathlessness and a productive cough. CXR demonstrated multiple lung cavities, and repeat CTPA reconfirmed extensive thromboembolism. After two weeks of antibiotic therapy, she underwent right heart catheterisation which confirmed CTEPH, with a mean PAP of 35 mmHg. Vasodilators were commenced and she is awaiting pulmonary endarterectomy.
Figure 5.
Extensive thrombus in the right main pulmonary artery on CTPA in patient with severe pulmonary hypertension.
Discussion
Pulmonary cavities form when necrotic lung parenchyma communicates with a patent airway. Most commonly they occur as abscesses in the context of infection, or in tumours. Other unusual associations include inflammatory causes e.g. rheumatoid arthritis and Wegeners granulomatosis, following trauma, and in pulmonary infarction when tissue necrosis is considered rare due to dual blood supply from both pulmonary and bronchial arteries. There are a few papers describing pulmonary cavities in the context of pulmonary thromboembolism. They all refer to patients with acute pulmonary embolism and are based on post-mortem studies combined with CXR and lung scintigraphy findings (16,17,18,20,21). As far as we are aware, studies on the incidence of pulmonary cavities in chronic thromboembolic pulmonary disease have not been previously reported in the literature.
Imaging findings in CTEPH are often non-specific. The CXR frequently appears normal. Abnormalities include enlarged pulmonary arteries, pruning of peripheral vessels, and pulmonary infarcts, which tend to be subpleural, peripheral, wedge-shaped areas of opacification. The most common radiological changes seen on CT are mosaic perfusion and micronodular disease (15), along with an enlarged main pulmonary artery and abrupt tapering of segmental vessels. Intimal covering of incompletely resolved thrombus leads to mural pulmonary arterial thrombus, which may calcify over time. The presence of mural thrombus results in increased right ventricular strain due to flow-obstruction and ultimately leads to right heart failure and death. (5,14).
In acute pulmonary embolism, lung infarction occurs in less than 15% of cases, and of these, only 5% cavitate (18). The prevalence of cavity formation as described from postmortem studies is 3.4% (22,23,24). More than 75% of cavities develop in the mid and upper zones (apical and posterior segments upper lobes and superior segments lower lobes), and they more commonly appear in the right lung. (16,17). This distribution is opposite to the common position of emboli and non-cavitary infarcts which occur more frequently in the lower zones, possibly due to higher blood flow there(19). Cavities are more commonly single than multiple (16), may be thin or thick-walled (25), and usually occur in the presence of infection (16,17). Cavitation in a sterile infarct is exceedingly uncommon, and other causes should be considered(16). The natural history of cavities seen in acute PE shows that some resolve, leaving a linear scar with associated pleural thickening, while others remain. Maturing cavities tend to enlarge with increasing uniformity and thinning of the wall before becoming smaller or resolving. Observation suggests that patients developing more than one cavity have an increased mortality, possibly as a reflection of disease severity (16).
In this cohort of 104 patients with CTEPH, 10.9% developed a cavity. 8 had a single cavity, 1 patient had 2 cavities and 2 patients developed 3 cavities. More cavities were found in the upper lobes (8), while 5 were in the lung bases and 3 in the apical segment of the lower lobe. Whilst these findings mirror the number of cavities and their location and distribution as described in acute thromboembolic disease, they also suggest that cavity formation is approximately three times more common than seen in acute PE (10.9% vs 3.4%). Most of the cavities had thick, well-defined walls and surrounding consolidation at diagnosis with one containing an air-fluid level. The two cavities without surrounding consolidation were found during routine imaging, so pre-existing consolidation was unknown. Follow-up imaging was performed in seven patients with a total of nine cavities. Surrounding consolidation resolved in all cases. During follow-up, the natural progression of the cavities in our CTEPH cohort mirrored that described in acute disease, with either complete or partial resolution and wall-thinning in most cases. Five of the nine cavities also showed an initial increase in size. Cavity diagnosis in seven patients followed an acute exacerbation of symptoms including increased breathlessness, cough and pyrexia, with the remaining cavities being diagnosed during routine workup. Three patients had positive sputum cultures and an elevated CRP, two patients had an elevated CRP with negative sputum culture, two patients had a normal CRP and no sputum culture, and the reminder had neither inflammatory markers nor sputum culture performed. Ten of the patients were treated with antibiotics with clinical or radiological improvement. One patient had normal CRP, sputum cultures were not obtained and antibiotics were not prescribed. The cavity resolved spontaneously, suggesting that this was a true sterile cavity.
Comparing thrombus position between patients with and without cavity formation, proximal mural thrombus was seen in 65 of the 93 patients who did not form a cavity (69.9%) compared to 8 of the 11 patients who did (72.2%). Conversely, 22 of the patients without a cavity had distal thrombus (23.7%) compared to three of the patients with (27.3%). Due to the small numbers involved, it was not possible to perform meaningful statistics on these figures, however, no major differences were observed. Six of the patients without a cavity did not have mural thrombus, implying that CTEPH was related to distal disease.
Cavity development was not associated with an increase in mortality (26.8% vs 27.3%). Of the three patients who died, one developed multiple cavities. However two other patients with multiple cavities remain alive, suggesting that development of multiple cavities is not necessarily a predictor of mortality.
Many features of the cavities occurring in our series of CTEPH patients concur with those reported in the literature in acute pulmonary embolism. However, our study suggests that cavity formation is 3 times more common in chronic thromboembolic disease, and all our cases were associated with pulmonary arterial thrombus and other evidence of severe disease. Whilst infection or inflammation was not proved in all cases, many of the cavities were diagnosed following an exacerbation of symptoms, with improvement following a course of antibiotics. Proximal or distal thrombus position does not seem to affect the likelihood of a cavity developing, and the presence of a cavity does not seem to be associated with an increased mortality. We therefore conclude that cavity formation is a recognised complication of CTEPH, and is more commonly encountered than in acute thromboembolic disease. Treatment with antibiotics is recommended regardless of sputum culture results and follow-up with serial CXR or low dose CT thorax should be performed to ensure partial or full resolution.
Teaching Point
Cavity formation is a recognised complication of CTEPH, which is more commonly encountered than in acute thromboembolic disease, and cavity presence may serve as an indicator of disease severity but not as a predictor of mortality.
Abbreviations
- CTEPH
chronic Thromboembolic pulmonary hypertension
- PH
Pulmonary hypertension
- PAP
pulmonary arterial pressure
- CXR
plain chest radiograph
- CTPA
computed tomography pulmonary angiogram
- HRCT
high resolution computed tompgraphy
- MRI
magnetic resonance imaging
- ISWT
incremental shuttle walk test
- CT
computed tomography
- BMI
body mass index
- CRP
c-reactive protein
- MRSA
methicillin resistant staphylococcus aureus
- MRPA
magnetic resonance pulmonary angiography
Footnotes
Authors' contributions
- guarantor of integrity of the entire study – EJR Van Beek
- study concepts – EJR van Beek, DG Kiely
- study design – n/a
- definition of intellectual content – EJR van Beek
- literature research – HJ Harris
- clinical studies – n/a
- experimental studies - n/a
- data acquisition – R Barraclough, I Armstrong, C Davies, EJR van Beek
- data analysis – HJ Harris
- statistical analysis – n/a
- manuscript preparation – HJ Harris
- manuscript editing – HJ Harris, EJR van Beek
- manuscript review – DG Kiely, EJR van Beek
References
- 1.Warrell D, Cox TM, Firth JD, Edward J. Oxford Textbook of Medicine. 2004. Section 15.52. [Google Scholar]
- 2.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 3.Grainger and Allison Diagnostic Radiology. A Textbook of Medical Imaging. (4th ed) 1:879. [Google Scholar]
- 4.Chazova I, Loyd JE, Zhdanov VS, et al. Pulmonary Artery Adventitial Changes and Venous Involvement In Primary Pulmonary Hypertension. American Journal of Pathology. 1995;146:389–97. [PMC free article] [PubMed] [Google Scholar]
- 5.D'Alonzo, et al. Survival in Patients With Primary Pulmonary Hypertension. Results From a National Prospective Registry. Annals of Internal Medicine. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, et al. Primary Pulmonary Hypertension: Natural History and The Importance of Thrombosis. Circulation. 1984;70:580–7. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 7.Rubin LJ. American College of Chest Physicians Diagnosis and Management of Pulmonary Arterial Hypertension: ACCP Evidence-Based Clinical Practical Guidelines. Chest. 2004;126( 1 suppl):7S–10S. doi: 10.1378/chest.126.1_suppl.7S. [DOI] [PubMed] [Google Scholar]
- 8.Roeleveld R, Marcus JT, Boonstra A, et al. A Comparison of MRI-Based Methods of Estimating Pulmonary Artery Pressure in Pulmonary Hypertension. J Magn Reson Imaging. 2005;22(1):67–72. doi: 10.1002/jmri.20338. [DOI] [PubMed] [Google Scholar]
- 9.Saba TS, Foster J, Cockburn M, Cowan M, Peacock AJ. Ventricular Mass Index Using Magnetic Resonance Imaging Accurately Estimates Pulmonary Artery Pressure. Eur Respir J. 2002;20(6):1519–24. doi: 10.1183/09031936.02.00014602. [DOI] [PubMed] [Google Scholar]
- 10.Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, Al-Nahhas A. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007 May;48(5):680–4. doi: 10.2967/jnumed.106.039438. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaou K, Schoenberg SO, et al. Pulmonary Arterial Hypertension: Diagnosis With Fast Perfusion MR Imaging and High-Spatial-Resolution MR Angiogrpahy. Preliminary Experience Radiology. 2005;236(2):694–703. doi: 10.1148/radiol.2361040502. [DOI] [PubMed] [Google Scholar]
- 12.Barst RJ, et al. A Comparison of Continuous Intravenous Epoprostenol (Prostacyclin) With Conventional Therapy For Primary Pulmonary Hypertension. The Primary Pulmonary Hypertension Study Group. NEJM. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 13.Channick RN, et al. Effects of the Dual Endothelin-Receptor Antagonist Bosentan in Patients with Pulmonary Hypertension: A Randomised Placebo-Controlled Study. Lancet. 2001;258:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 14.Rozkovec A, Montanes P, Oakley C. Factors That Influence the Outcome of Primary Pulmonary Hypertension. British Heart Journal. 1986;55:449–58. doi: 10.1136/hrt.55.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansell DM. Small Vessel Diseases of the Lung: CT-Pathologic Correlates. Radiology. 2002;225:639–653. doi: 10.1148/radiol.2253011490. [DOI] [PubMed] [Google Scholar]
- 16.Wilson AG, Joseph AEA, Butland RJA. The Radiology of Aseptic Cavitation in Pulmonary Infarction. Clin Rad. 1986;37:327–333. doi: 10.1016/s0009-9260(86)80263-x. [DOI] [PubMed] [Google Scholar]
- 17.Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary Cavitation Following Pulmonary Infarction. Medicine (Baltimore) 1985;64(5):342–348. doi: 10.1097/00005792-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Morgenthaler TI, Ryu JH, Utz JP. Cavitary Pulmonary Infarct in Immunocompromised Hosts. Mayo Clin Proc. 1995 Jan;70(1):66–68. doi: 10.1016/S0025-6196(11)64668-5. [DOI] [PubMed] [Google Scholar]
- 19.Smith GT, Dexter L, Dammin GL. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, editors. Pulmonary Embolic Disease. pp. 120–130. [Google Scholar]
- 20.Redline S, Tamashefski JF, Jr, Altose MD. Cavitating lung Infarction after Bland Pulmonary Thromboembolism In Patients With The adult Respiratory Distress Syndrome. Thorax. 1985;40(12):915–9. doi: 10.1136/thx.40.12.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao YQ, Jin LR, Chen WC. Pulmonary Infarction Presenting As Asceptic Cavitation. Chin Med J (Engl) 1990;103(8):689–91. [PubMed] [Google Scholar]
- 22.Chester EM, Krausse GR. Lung Abscess Secondary To Asceptic Pulmonary Infarction. Radiology. 1942;39:647–654. [Google Scholar]
- 23.Murray G, MacKenzie R. Postoperative Thrombosis And Embolism American. Journal of Surgery. 1942;57:414–428. [Google Scholar]
- 24.Levin L, Kernohan JW, Moersch HJ. Pulmonary Abcess Secondary To Bland Pulmonary Infarction. Diseases of the Chest. 1948;14:218–232. doi: 10.1378/chest.14.2.218. [DOI] [PubMed] [Google Scholar]
- 25.Felson B. Chest Roentgenology. (2nd Ed) 1978:319–321. [Google Scholar]